Method for preparing 2,5-furandicarboxylicacid
A technology of furandicarboxylic acid and catalyst, applied in the direction of organic chemistry, etc., can solve the problems of poor solubility and instability of 5-hydroxymethylfurfural, achieve high reaction efficiency, promote hydrolysis isomerization process, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
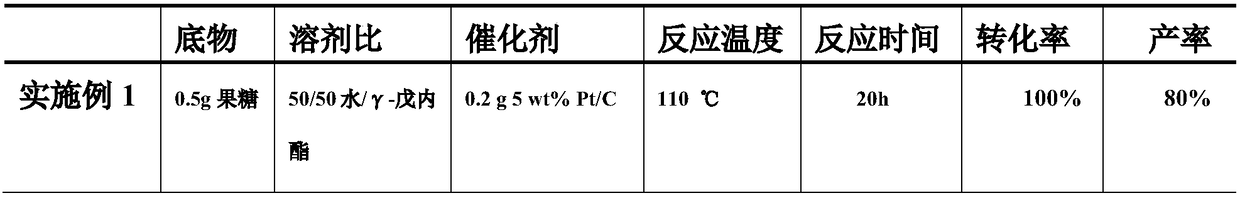
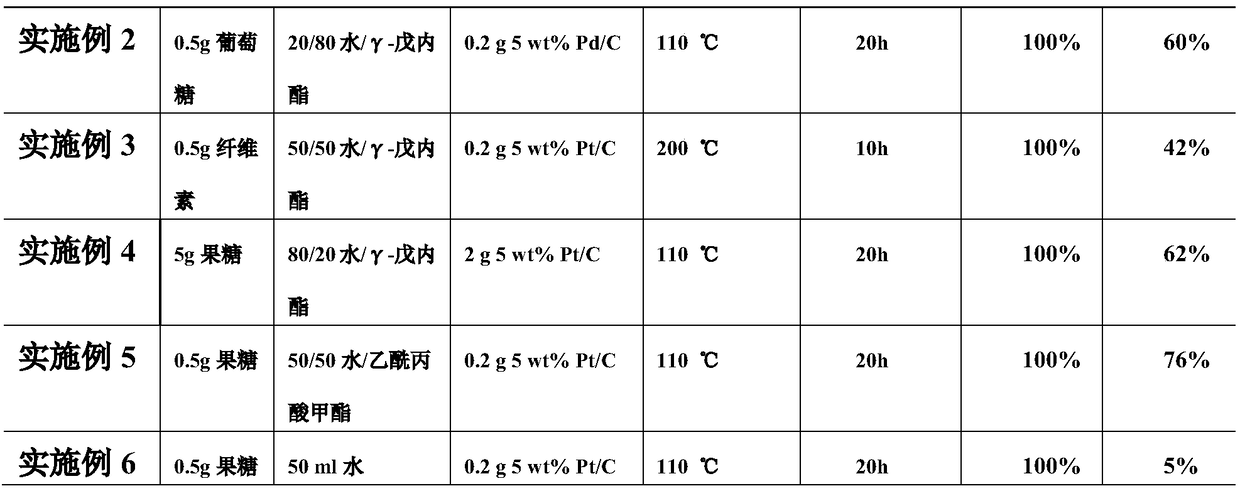
Embodiment 1
[0023] Measure 50ml of water and 50ml of gamma-valerolactone in a 250ml reactor, add 0.5g of fructose and 0.2g of 5wt% Pt / C catalyst, fill the reactor with 4MPa oxygen, rise to 110°C at 5°C / min, and Stir continuously, react for 20 hours, recrystallize after the reaction is completed and cool down to room temperature, and obtain 0.4 g of the target product 2,5-furandicarboxylic acid.
Embodiment 2
[0025] Measure 20ml of water and 80ml of γ-valerolactone in a 250ml reactor, add 0.5g of glucose and 0.2g of 5wt% Pd / C catalyst, fill the reactor with 4MPa oxygen, raise it to 110°C at 5°C / min, and Stir continuously, react for 20 hours, recrystallize after the reaction is completed and cool down to room temperature, and obtain 0.3 g of the target product 2,5-furandicarboxylic acid.
Embodiment 3
[0027] Measure 50ml of water and 50ml of γ-valerolactone in a 250ml reactor, add 0.5g of cellulose and 0.2g of 5wt% Pt / C catalyst, fill the reactor with 4MPa oxygen, and raise it to 200°C at 5°C / min. Stir continuously, react for 10 h, recrystallize after the reaction is completed and cool down to room temperature, and obtain 0.21 g of the target product 2,5-furandicarboxylic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com