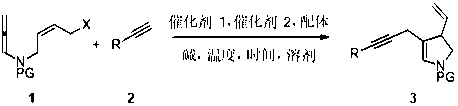Method for synthesizing 3-vinyl-4-acetenyl-2,3-dihydropyrrole derivative
A technology of dihydropyrrole and ethynyl, which is applied in the direction of silicon organic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the problems of harsh reaction conditions and unsatisfactory yields, etc. Achieve the effect of stable performance, low cost and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] figure 2 ;
[0044] In a 25 mL reaction tube, the PdCl 2 (PPh 3 ) 2 (0.02 mmol), CuCl ((0.01 mmol)), K 3 PO 4 ·3H 2 O (0.4 mmol) was put in, airtight, and replaced with nitrogen three times; allenamine substrate 1a (0.2 mmol) was dissolved in dehydrated dioxane solvent (2.0 mL), and then, the solution was injected into In a closed reaction test tube; weigh the ethynyl derivative 2a (0.4mmol), at 80 o C temperature, slowly, drop by drop into the closed reaction test tube, which lasted 2.5 hours; after TLC detection, the system was cooled to room temperature; washing, extraction of organic phase, thickened silica gel spin-drying; fast column chromatography After passing the target product, 3aa (85%) was obtained as a yellow-brown liquid; 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.59 (d, J = 8.0 Hz,2H), 7.30–7.21 (m, 7H), 6.34 (s, 1H), 5.26–5.17 (m, 1H), 4.94–4.88 (m, 2H),3.66 (t, J = 10.0 Hz, 1H), 3.29–3.17 (m, 2H), 2.98 (d, J = 19.6 Hz, 1H), 2.85(d, J = 20.0 Hz...
Embodiment 2
[0046] image 3 ;
[0047] In a 25 mL reaction tube, Pd(PPh 3 ) 4 (0.02 mmol), CuCl ((0.01 mmol)), K 3 PO 4 ·3H 2 O (0.4 mmol) was put in, airtight, and replaced with nitrogen three times; allenamine substrate 1b (0.2 mmol) was dissolved in dehydrated dioxane solvent (2.0 mL), and then the solution was injected into In a closed reaction test tube; weigh the ethynyl derivative 2b (0.4mmol), at 80 o C temperature, slowly, drop by drop into the closed reaction test tube, which lasted 2.5 hours; after TLC detection, the system was cooled to room temperature; washing, extraction of organic phase, thickened silica gel spin-drying; fast column chromatography The target product was passed through to obtain brown liquid 3bb (82%); 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.68 (d, J = 7.6 Hz, 2H),7.34–7.28 (m, 4H), 7.12 (d, J = 7.6 Hz, 2H), 6.42 (s, 1H), 5.36–5.27 (m, 2H),5.03–4.98 (m, 2H), 3.76 (t, J =10.0 Hz, 1H), 3.39–3.26 (m, 2H), 3.07 (d, J =19.6 Hz, 1H), 2.94 (d, J = 2...
Embodiment 3
[0049] Figure 4 ;
[0050] In a 25 mL reaction tube, the PdCl 2 (PPh 3 ) 2 (0.02 mmol), CuCl ((0.01 mmol)), K 3 PO 4 (0.4mmol) into it, seal it, and replace it with nitrogen for 3 times; dissolve allenamine substrate 1c (0.2 mmol) in dioxane solvent (2.0 mL) except water, and then put the solution into the airtight In the reaction test tube; weigh the ethynyl derivative 2c (0.4 mmol), at 80 o C temperature, slowly, drop by drop into the closed reaction test tube, which lasted 2.5 hours; after TLC detection, the system was cooled to room temperature; washing, extraction of organic phase, thickened silica gel spin-drying; fast column chromatography The target product was passed through to obtain 3cc (77%) of yellow-brown liquid; 1 H NMR (400 MHz, CDCl 3 , δ ppm): 7.68 (d, J = 7.6 Hz, 2H), 7.33(d, J = 8.0 Hz, 4H), 6.84 (d, J = 8.0 Hz, 2H), 6.42 (s, 1H), 5.35-5.27 (m,1H), 5.03-4.98 (m, 2H), 3.81 (s, 3H), 3.75 (t, J = 10 Hz, 1H), 3.38-3.26 (m,2H), 3.06 (d, J = 19...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com