Novel PD-1 tumor immunosuppressant and drug preparation method thereof
An immunosuppressant, PD-1 technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of limited reports and embarrassment of PD-1 inhibitors, achieve inhibition of cancer cell immunity and humoral immunity, improve The effect of killing ability and improving antigen phagocytosis ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
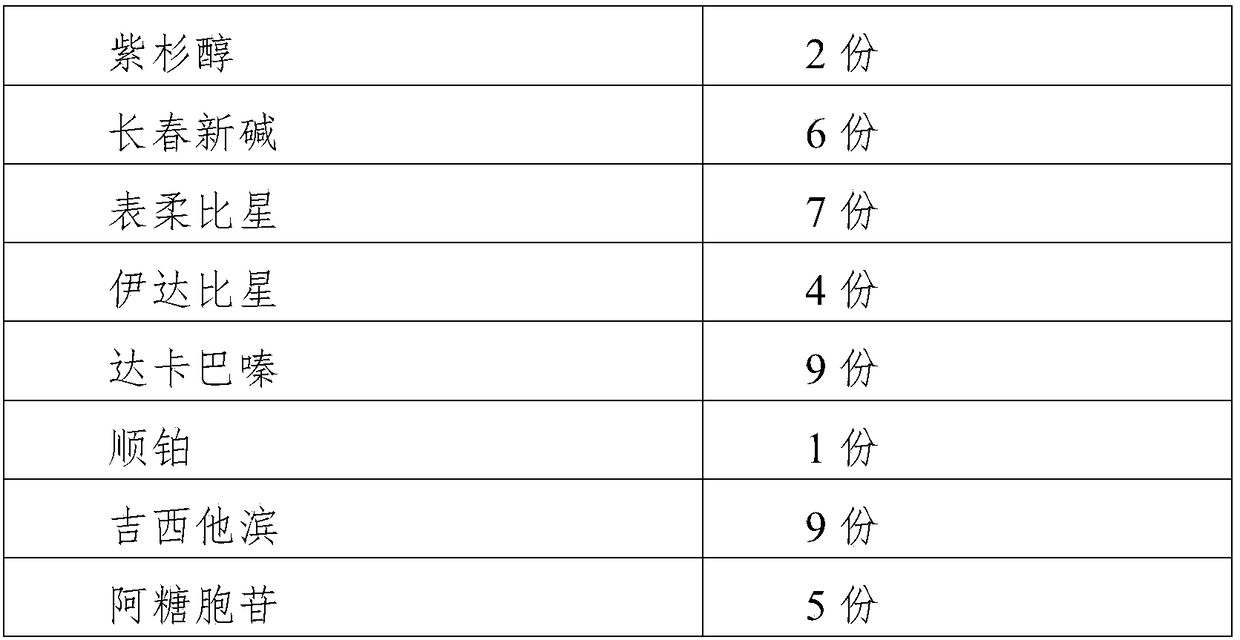
5 copies
[0033] The preparation method of the immunosuppressant medicine comprises the following steps:
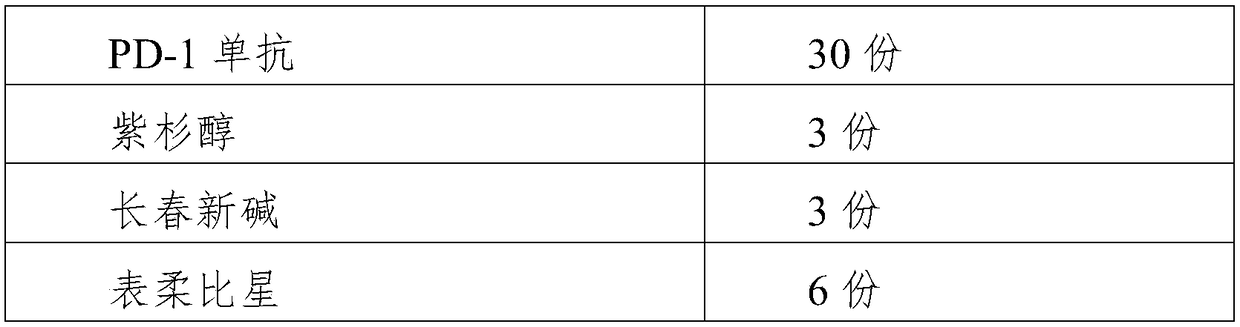
[0034] (1) Add the PD-1 monoclonal antibody into a glass container, add a small amount of water, adjust the solution concentration to 250 g / ml with sodium carbonate buffer solution with a pH of 9.0, dialyze overnight at a low temperature of 1°C, and then dissolve the PD-1 monoclonal antibody Anti-transfer into a shock container, avoid light and shake for 4 hours, and store it at -20°C for standby;
[0035] (2) Take the metabolic antagonist, disperse it in 30ml of chloroform, add alkaloid, incubate at room temperature for 25min, then add platinum agent, stir magnetically at a constant temperature at room temperature, add dropwise 10% ammonia water to adjust the pH to 9.2, Continue to stir for 10 minutes, then transfer to a water bath on a rotary evaporator to 80°C, drain the air in the bottle and continue to rotate, remove the organic phase, and set aside;
[0036]...
Embodiment 2
[0038]
[0039]
[0040] The preparation method of the immunosuppressant medicine comprises the following steps:
[0041] (1) Add PD-1 monoclonal antibody into a glass container, add a small amount of water, adjust the solution concentration to 200g / ml with sodium carbonate buffer solution with a pH of 9.2, dialyze overnight at 4°C, and then dissolve PD-1 monoclonal antibody Anti-transfer into a shock container, avoid light and shake for 3 hours, and store it at -20°C for standby;
[0042](2) Take the metabolic antagonist, disperse it in 30ml of chloroform, add alkaloid, incubate at room temperature for 30min, then add platinum agent, stir magnetically at a constant temperature at room temperature, add dropwise 10% ammonia water to adjust the pH to 9.0, Continue to stir for 15 minutes, then transfer to a water bath on a rotary evaporator to 80°C, drain the air in the bottle and continue to rotate, remove the organic phase, and set aside;
[0043] (3) Take the material o...
Embodiment 3
[0045]
[0046]
[0047] The preparation method of the immunosuppressant medicine comprises the following steps:
[0048] (1) Add PD-1 monoclonal antibody into a glass container, add a small amount of water, adjust the solution concentration to 250g / ml with sodium carbonate buffer solution with a pH of 9.0, dialyze overnight at 2°C, and then dissolve PD-1 monoclonal antibody Anti-transfer into a shock container, avoid light and shake for 4 hours, and store it at -20°C for standby;
[0049] (2) Take the metabolic antagonist, disperse it in 30ml of chloroform, add alkaloid, incubate at normal temperature for 25min, then add platinum agent, stir magnetically at constant temperature at room temperature, add dropwise 10% ammonia water to adjust the pH to 9.0, Continue to stir for 15 minutes, then transfer to a water bath on a rotary evaporator to 80°C, drain the air in the bottle and continue to rotate, remove the organic phase, and set aside;
[0050] (3) Take the material ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com