Recombinant human growth hormone sterile powder for injection and preparation method thereof
A technology of human growth hormone and sterile powder, which is applied in the field of biochemistry, can solve the problems of loss of biological activity and reduction, and achieve the effects of ensuring stability and activity, good transportation stability, and good acceleration stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
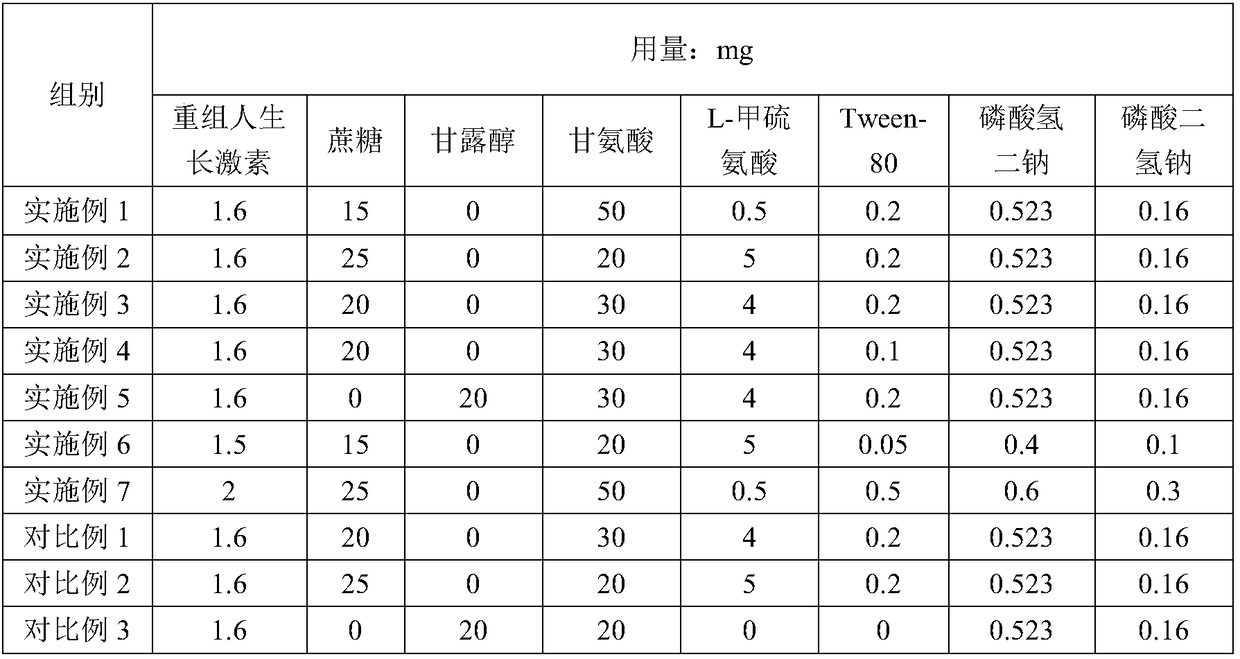
[0029] A recombinant human growth hormone sterile powder for injection, the composition of which is shown in Table 1;
[0030] The sterile powder of recombinant human growth hormone for injection is prepared by the following steps:
[0031] 1. Weighing:
[0032] Take a beaker and put it on the balance to peel it, weigh the auxiliary materials according to the above dosage, seal them with tin foil, put them aside, and continue to weigh the next auxiliary materials;
[0033] 2. Dissolving:
[0034] Each excipient is dissolved separately, stirred, and after all the excipients are clarified, the excipients are mixed in turn, and rinsed with a small amount of water for injection; add recombinant human growth hormone stock solution, and constant volume;
[0035] 3. Filter:
[0036] Connect the pipeline, and start filtering after checking that it is correct, and the filtering time should not exceed 30 minutes;
[0037] 4. Filling:
[0038] Assemble the needle and tubing, turn on...
Embodiment 2
[0048] A recombinant human growth hormone sterile powder for injection, the composition of which is shown in Table 1;
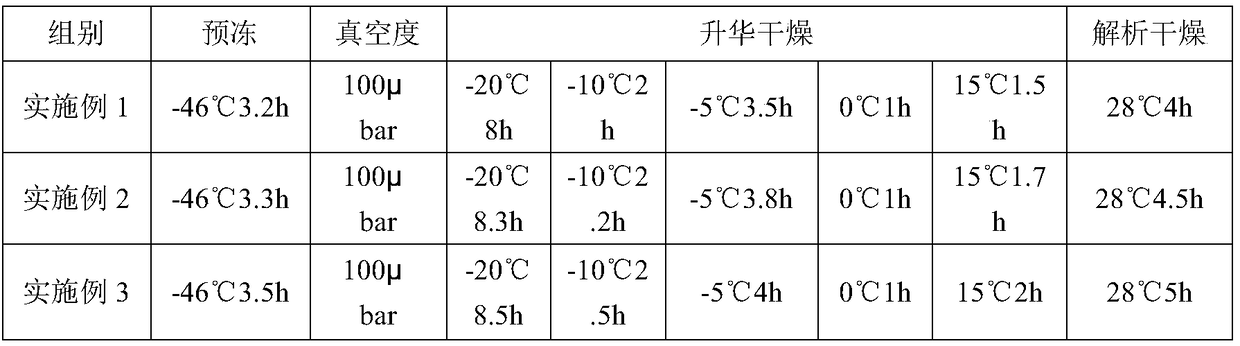
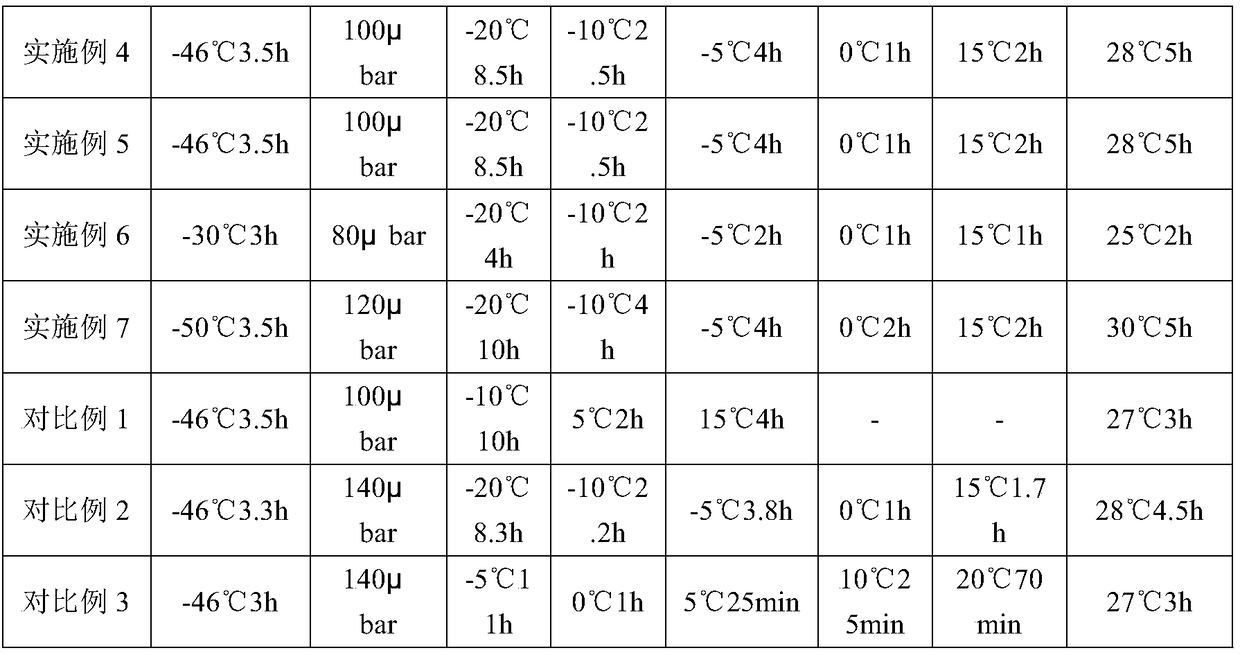
[0049] See Example 1 for the preparation method, and see Table 2 for specific parameters.
Embodiment 3
[0051] A recombinant human growth hormone sterile powder for injection, the composition of which is shown in Table 1;
[0052] See Example 1 for the preparation method, and see Table 2 for specific parameters.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com