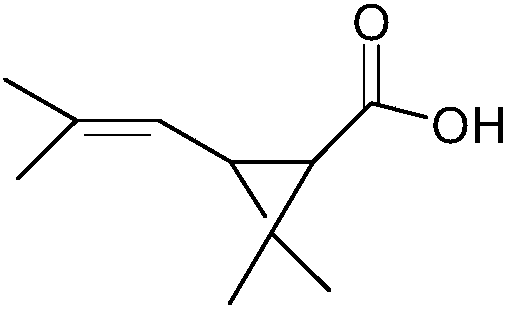
Preparation method of dextro trans-chrysanthemic acid
A technology for the first chrysanthemic acid ester and chrysanthemic acid, which is applied in the field of preparation of the first dextrorotatory trans chrysanthemic acid, can solve the problems of increasing manufacturing steps, and the practical application of production is not large, so as to facilitate recycling and improve hydrolysis The effect of single selectivity and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method of the first dextrorotatory trans-type chrysanthemic acid: 200 g of ethyl chrysanthemic acid (cis / trans = 10 / 90), 1000 g of 1% ammonia water, and 1 g of EYN045 esterase (US Sigma) were put into a 1000 ml four-neck bottle , stirred and reacted at 40°C for 48 hours, after the reaction, the oil-water layer was separated (centrifugation or ultrafiltration membrane separation was used to recover the enzyme and the raw material was recycled), the water layer was split and concentrated hydrochloric acid was added to adjust the pH of the system to 2, and the solvent toluene was added to extract the dextrorotational For trans chrysanthemic acid, the solvent is removed by precipitation of the extract to obtain about 67.5 g of the desired product, the first dextrorotatory chrysanthemic acid, the gas chromatography capillary column analysis content is 99.52%, and the gas chromatography chiral column analysis dextrorotation trans isomer content is 99.21%.
Embodiment 2
[0022] A preparation method for the first dextrorotatory trans-type chrysanthemic acid: 200 g of methyl chrysanthemic acid (cis / trans = 10 / 90), 1000 g of 1% ammonia solution, 1 g of EYN045 esterase (US Sigma ), stirred and reacted at 40°C for 48hr, after the reaction was completed, the oil-water layer was separated according to the operation in Example 1, and the water layer was acidified, extracted, and desolvated to obtain about 68.9g of D-trans chrysanthemic acid, and the gas chromatography capillary column analysis content was 99.42% , gas chromatography chiral column analysis of the right-handed trans-isomer content was 99.01%.
Embodiment 3
[0024] A preparation method of the first dextrorotatory trans-type chrysanthemic acid: 200 g of isopropyl chrysanthemic acid (cis / trans = 10 / 90), 1000 g of 1% ammonia solution, and 1 g of EYN045 esterase are put into a 1000 ml four-necked bottle. Sigma), stirred and reacted at 40°C for 48hr, after the reaction was completed, the oil-water layer was separated according to the operation in Example 1, and the water layer was acidified, extracted, and desolvated to obtain about 65g of D-trans chrysanthemic acid, and the gas chromatography capillary column analysis content was 99.55% , gas chromatography chiral column analysis of the right-handed trans-isomer content was 99.51%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com