Quality control method of danggui sini decoction composition
A quality control method, the technology of Sini Decoction, is applied in the quality standard detection of traditional Chinese medicine compound granules, and in the field of quality control of Danggui Sini Decoction compositions, which can solve the problems of many components, single quality control, few indicators, etc., and reach an objective conclusion. , control the quality of the preparation, the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1. The preparation of Angelica Sini Decoction composition
[0062] Take Angelica 375g; Guizhi 375g; Paeoniae Alba 375g; Asarum 125g; Radix Glycyrrhiza 250g; Filtrate, concentrate the filtrate to a clear paste with a relative density of 1.20-1.25 (60° C.), add an appropriate amount of maltodextrin, mix well, dry, and granulate to make 1000 g to obtain the composition of Angelica Sini Decoction.
Embodiment 2
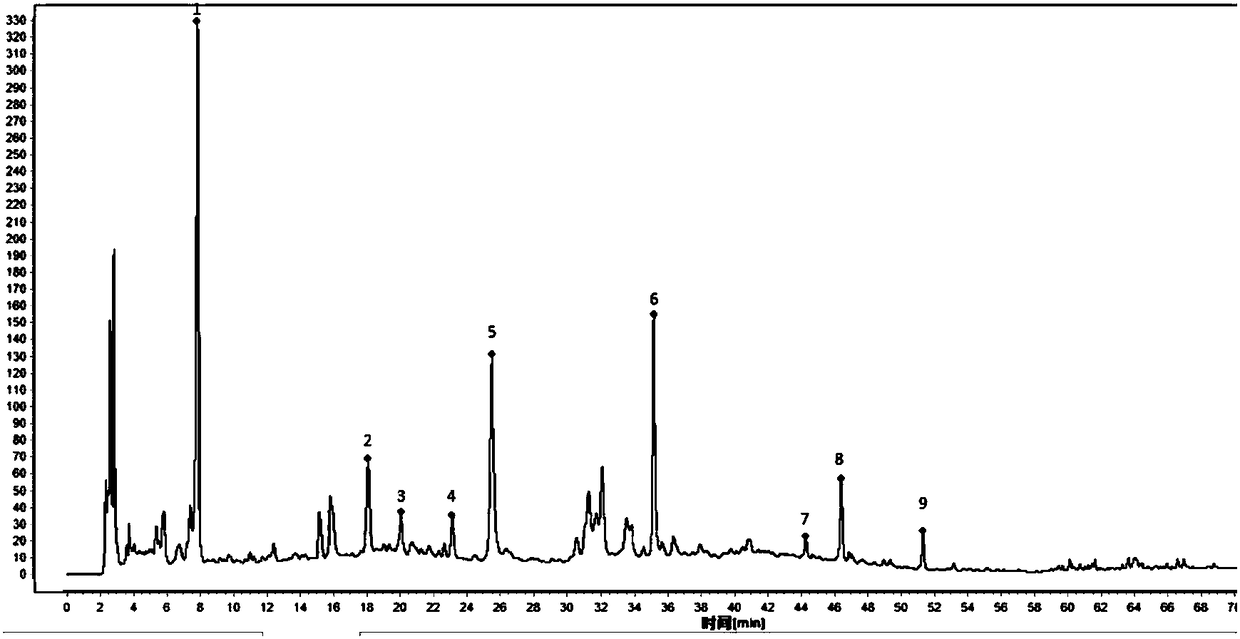
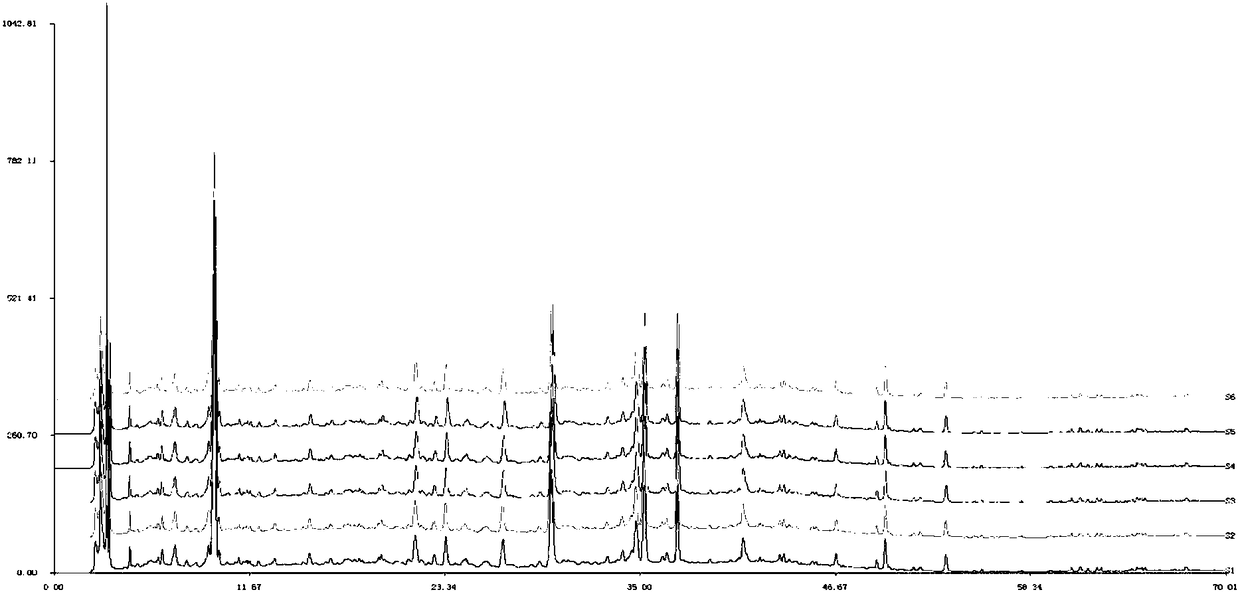
[0063] Example 2. Research on Fingerprint of Danggui Sini Decoction Composition
[0064] Instruments and reagents
[0065] Chromatography instrument: American Agilent 1100 series (G1322A online vacuum degasser, G1311A quaternary gradient pump, G1313A autosampler, G1316A column thermostat, G1315B diode array detector);
[0066] Chromatographic column: Kromasil 100-5-C18 4.6×250mm 5μm;
[0067] Reagents: methanol, Tianjin Damao Chemical Reagent Factory, chromatographically pure, batch number: 20160310; acetonitrile, Tianjin Biaoshiqi Technology Development Co., Ltd., chromatographically pure, batch number: AC-12060107; phosphoric acid, Tianjin Tongguang Fine Chemical Company, analysis Pure, batch number: 20150712; water, Millipore ultrapure water;
[0068] Danggui Sini Decoction Composition Medicinal Materials: Three batches of Danggui Sini Decoction composition 160501, 160502, 160504 were provided by China Resources Sanjiu Group for trial production according to the steps of ...
Embodiment 3
[0109] Example 3. Determination of Angelica, Guizhi, Radix Glycyrrhiza and Radix Paeoniae Alba in the composition of Danggui Sini Decoction by HPLC
[0110] Instruments and reagents:
[0111] instrument:
[0112] High performance liquid chromatography Agilent 1100 chromatographic system, including quaternary pump, automatic sampler, diode array detector, chromatographic workstation;
[0113] Test drug:
[0114] Acetonitrile was chromatographically pure from Tianjin Biaoshiqi Technology Development Co., Ltd.; phosphoric acid was analytically pure from Beijing Beihua Fine Chemicals Co., Ltd.; water was self-made from double distilled water.
[0115] The reference substance of paeoniflorin was purchased from the China Institute for the Control of Pharmaceutical and Biological Products, which met the requirements of the reference substance for content determination. Batch number 110736-201604; Liquiritin reference substance, purchased from China Institute for the Control of Pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com