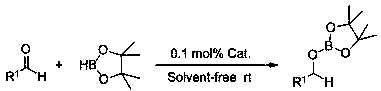Method for preparing borate from aliphatic aldehyde
A technology for boronate esters and fatty aldehydes, which is applied in the field of preparing boronate esters, can solve the problems of weakening the nucleophilic addition activity of carbonyl groups, weakening the positive charge of carbonyl carbonyls, reducing the activity of carbonyl groups, etc., and achieves simple and controllable reactions, good generalization, etc. adaptability, and the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
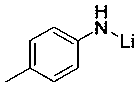
[0024] Example 1: Hydroboration reaction of cyclohexylbenzaldehyde and pinacol borane catalyzed by p-methylanilide lithium
[0025] In the reaction flask that has been dehydrated and deoxygenated, add 20ul of tetrahydrofuran solution (0.05M) of p-methylanilinide lithium under the protection of argon (0.1 mol% dosage, the same below), then add 0.1596 mL of borane with a syringe, mix well , then add 0.095 mL of 2-pyridinecarbaldehyde with a syringe, and the mixture is stirred at room temperature. After 10 min of reaction, the NMR yield is 99%, and then a small amount of tetrahydrofuran and excess borane are removed under reduced pressure to obtain the corresponding pinacol boron Ester C 6 h 5 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 8.61 (d, J = 5.4 Hz, 1H, Ar-H), 7.91 (t, J = 7.7 Hz, 1H, Ar-H), 7.49-7.41 (m, 2H, Ar-H), 5.10 (s, 2H, OCH 2 ), 1.32 (s,12H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 159.82 (Ar-C), 143.72 (Ar-C), 139.56(Ar-C), 123....
Embodiment 2
[0026] Example 2: Lithium p-methylanilide catalyzes the hydroboration reaction of propionaldehyde and pinacol borane
[0027] In the reaction flask that has been dehydrated and deoxygenated, under the protection of argon, add 20ul of tetrahydrofuran solution (0.05M) of p-methylanilinide lithium (0.1 mol% dosage), then add 0.1596 mL of borane with a syringe, mix well, and then use 0.072 mL of propionaldehyde was added into the syringe, and the mixture was stirred at room temperature. After 10 min of reaction, the NMR yield was 99%. After that, a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate CH 3 CH 2 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 HNMR (400 MHz, CDCl 3 ) δ 3.80 (t, J = 6.6 Hz, 2H, OCH 2 ), 1.63-1.54 (m, 2H, CH 2 ),1.25 (s, 12H, CH 3 ), 0.91 (t, J = 7.4 Hz, 3H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ82.04 (OC), 66.02 (OCH 2 ), 24.14 (CH 3 CH 2 ), 24.05 (CH 3 ), 9.55 (CH ...
Embodiment 3
[0028] Example 3: Hydroboration reaction of n-heptanal and pinacol borane catalyzed by p-methylanilide lithium
[0029] In the reaction flask that has been dehydrated and deoxygenated, under the protection of argon, add 20ul of tetrahydrofuran solution (0.05M) of p-methylanilinide lithium (0.1 mol% dosage), then add 0.1596 mL of borane with a syringe, mix well, and then use 0.1392 mL of n-heptanal was added into the syringe, and the mixture was stirred at room temperature. After 10 min of reaction, the NMR yield was 99%. After that, a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate C 6 h 13 COCH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). 1 H NMR (400 MHz, CDCl 3 ) δ 3.82 (t, J = 8Hz, 2H, OCH 2 ), 1.52-1.58 (m, 2H,CH 2 ), 1.27-1.34 (m, 8H, CH 2 ), 1.24 (s, 12H, CH 3 ), 0.87 (t, J = 8 Hz, 3H, CH 3 ). 13 C NMR (100 MHz, CDCl 3 ) δ 82.02 (OC), 64.40 (OCH 2 ), 31.29 (CH 2 ), 30.92 (CH 2 ),28....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com