Preparation method of decarbamoyl cefuroxime
A technology of carbamoyl head and amino cephalosporanic acid is applied in the field of preparation of decarbamoyl cefuroxime, can solve the problems of reducing chemical reaction process, harsh temperature conditions, long production cycle and the like, and achieves production equipment and operation protection. Low requirements, mild reaction conditions, and the effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
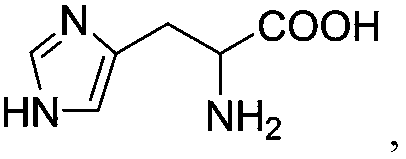
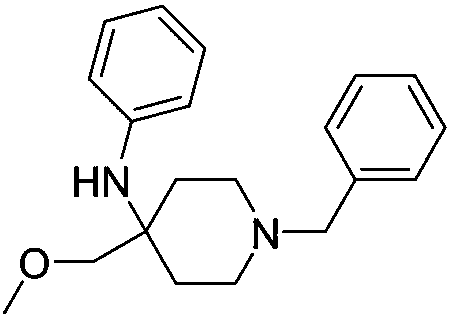
[0032] Add 150 mL of purified water to the reaction flask, and add 27.2 g of 7-aminocephalosporanic acid, 2 g of sodium hydroxide, 2.79 g of 2-amino-3-(4-imidazolyl)propionic acid and 0.62 g of 1-benzyl-4- (Methoxymethyl)-N-phenylpiperidin-4-amine, and cooled to 0°C, reacted under stirring, and monitored the reaction by HPLC to obtain 3-deacetyl-7-amino-cephalosporanic acid solution;
[0033] Add 31.2g of phosphorus pentachloride to another reaction flask, and add 90mL of methylene chloride, add 27.9g of 2-methoxyimino-2-furan ammonium acetate (SMIA) under stirring, and control the temperature at 25°C. Reacted for 0.5 hours, and the end point of the reaction was monitored by HPLC to obtain an acid chloride solution;
[0034] Add half of the obtained acid chloride solution to the 3-deacetyl-7-amino-cephalosporanic acid solution, add 4.1g of sodium acetate, stir and react, then add the remaining acid chloride solution to the reaction system, and then add 4.1g of acetic acid Sod...
Embodiment 2
[0037] Add 150 mL of purified water to the reaction flask, and add 27.2 g of 7-aminocephalosporanic acid, 2 g of sodium hydroxide, 1.35 g of 2-amino-3-(4-imidazolyl)propionic acid and 0.4 g of 1-benzyl-4- (Methoxymethyl)-N-phenylpiperidin-4-amine, and cooled to 10°C, reacted under stirring, and monitored the reaction by HPLC to obtain 3-deacetyl-7-amino-cephalosporanic acid solution;
[0038] Add 31.2g phosphorus pentachloride in another reaction flask, and add 90mL redistilled dichloromethane, add 2-methoxyimino-2-furan ammonium acetate (SMIA) 27.9g under stirring, control temperature is 30 ℃ for 0.5 hours, and the end point of the reaction was monitored by HPLC to obtain an acid chloride solution;
[0039] Add half of the obtained acid chloride solution to the 3-deacetyl-7-amino-cephalosporanic acid solution, add 5.7g of sodium acetate, stir the reaction, then add the remaining acid chloride solution to the reaction system, and then add 5.7g of acetic acid Sodium, continue ...
Embodiment 3
[0042] Add 150 mL of purified water to the reaction flask, and add 27.2 g of 7-aminocephalosporanic acid, 2 g of sodium hydroxide, 2.79 g of 2-amino-3-(4-imidazolyl)propionic acid and 0.62 g of 1-benzyl-4- (Methoxymethyl)-N-phenylpiperidin-4-amine, and cooled to 5° C., reacted under stirring, and monitored the reaction by HPLC to obtain 3-deacetyl-7-amino-cephalosporanic acid solution;
[0043] Add 31.2g of phosphorus pentachloride to another reaction flask, and add 90mL of methylene chloride, add 27.9g of 2-methoxyimino-2-furan ammonium acetate (SMIA) under stirring, and control the temperature at 20°C. Reacted for 0.5 hours, and the end point of the reaction was monitored by HPLC to obtain an acid chloride solution;
[0044] Add half of the obtained acid chloride solution to the 3-deacetyl-7-amino-cephalosporanic acid solution, add 5.7g of sodium acetate, stir the reaction, then add the remaining acid chloride solution to the reaction system, and then add 5.7g of acetic acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com