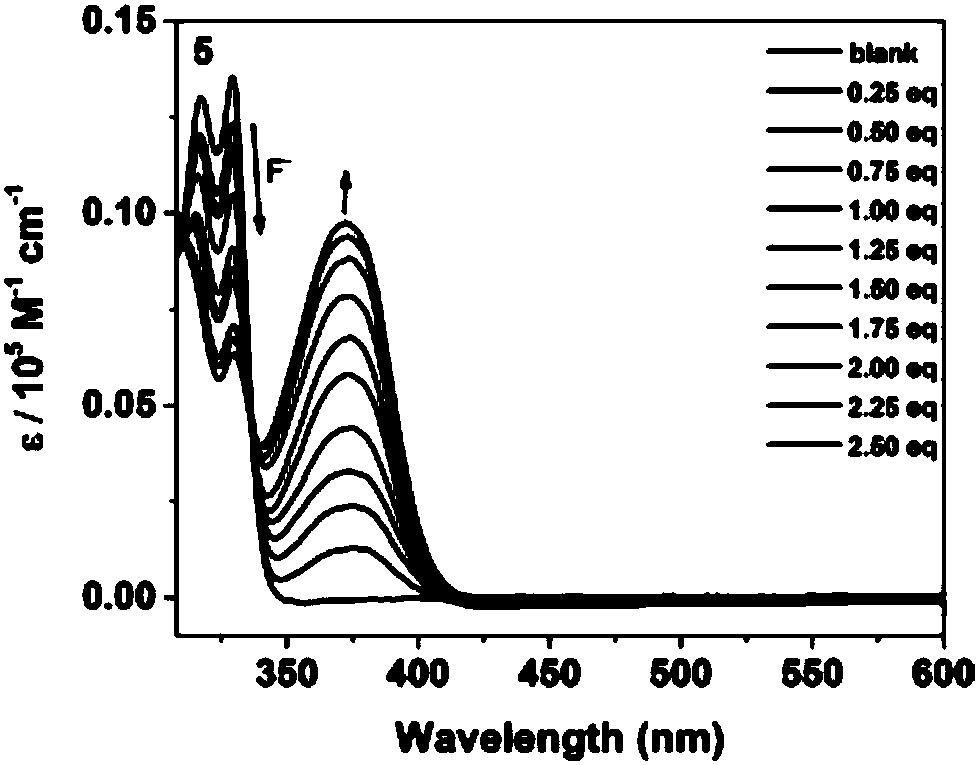
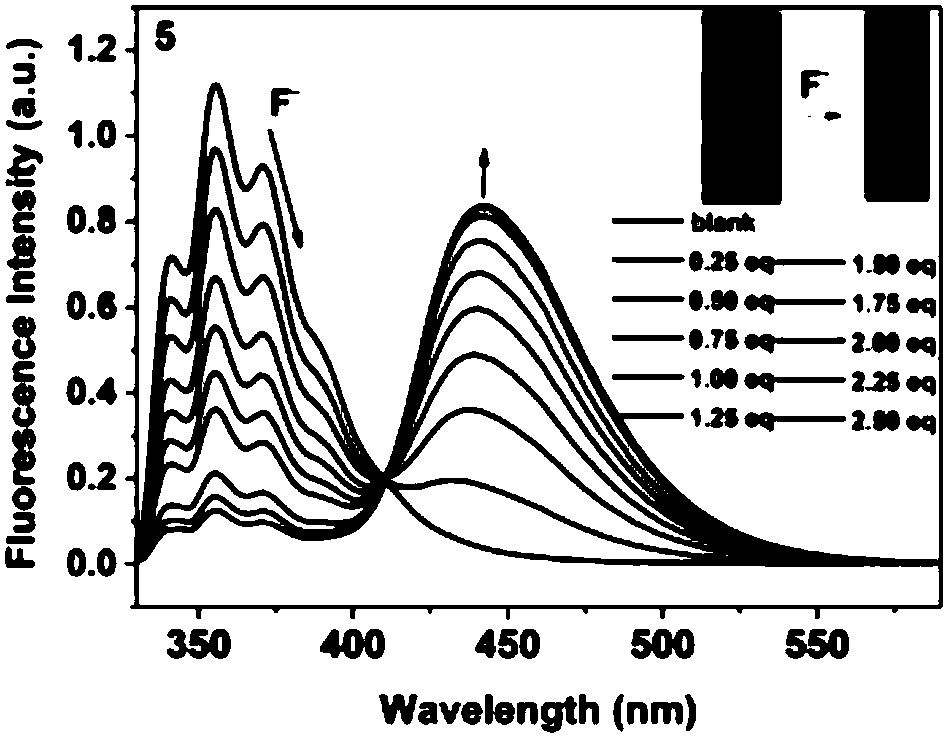
Preparation method of boron-nitrogen-containing small molecules and conjugated macromolecules and application in fluorine ion sensing
A technology of conjugated polymers and small molecules, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, analytical materials, etc., to achieve good selectivity, sensitive detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] 1. Preparation of boron nitrogen (BN) compound
[0064] (1) Preparation of Compound A
[0065] Under the protection of argon, dissolve 4,4-dibromobiphenyl in absolute anhydrous ether and place it in a Shrek reaction bottle, slowly add n-butyllithium dropwise at -78°C, after the dropwise addition, slowly raise the temperature Keep it at room temperature for 3 hours, then place it at -78°C, dissolve dimethyl tin dichloride in absolute anhydrous ether and slowly add it dropwise to the above solution, slowly warm up to room temperature, continue stirring for 12 hours, and the reaction is complete. Remove the solvent under reduced pressure, add n-hexane and heat to dissolve at 40°C, let it stand for 30 minutes, filter with celite, and remove the solvent under reduced pressure to obtain the target product.
[0066] Its reaction equation is as follows:
[0067]
[0068] (2) Preparation of compound B
[0069] Under the protection of argon, dissolve the tin-containing comp...
Embodiment 1
[0088] (1) Synthesis of compound 1
[0089] Under the protection of argon, dissolve 2,2'-dibromobiphenyl (1g, 3.2mmol) in 30mL of absolute anhydrous ether, place it in a Shrek reaction bottle at -78°C, and slowly add n-butyl Lithium 2.56mL (2.5M, 6.4mmoL), after the dropwise addition is completed, slowly warm up to room temperature and keep for three hours, then place it at -78°C, dissolve dimethyl tin dichloride (0.71g, 3.2mmol) in Slowly add dropwise absolute anhydrous ether to the above solution, slowly raise the temperature to room temperature, and continue to stir for 12 hours. After the reaction is completed, remove the solvent under reduced pressure, add n-hexane to dissolve by heating at 40°C, let it stand for 30 minutes, and filter with diatomaceous earth. The solvent was removed under reduced pressure to obtain the target product.
[0090] (2) Synthesis of compound 2
[0091] Under the protection of argon, dissolve 2,2'-diiodo4,4'-dibromobiphenyl (1.685g, 3.0mmol) ...
Embodiment 2
[0111] According to the method for the synthesis of compound 2 in Example 1, the 2,2'-diiodo4,4'-dibromodiphenyl side chain substituent dibromine is replaced by Cl, and other steps in this step are the same as those in the corresponding Example 1 ( The methods of 2)(4)(6)(7) are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com