Synthesis of optically-pure sulfur-containing quaternary heterocyclic drug intermediate
A compound, a sulfolane-based technology, is applied in the synthesis of sulfur-containing four-membered heterocyclic drug intermediates, which can solve the problems of danger, difficulty in operability, low overall synthesis yield, harsh reaction conditions, etc., and achieve optical purity of the product Good, simple equipment and process, high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
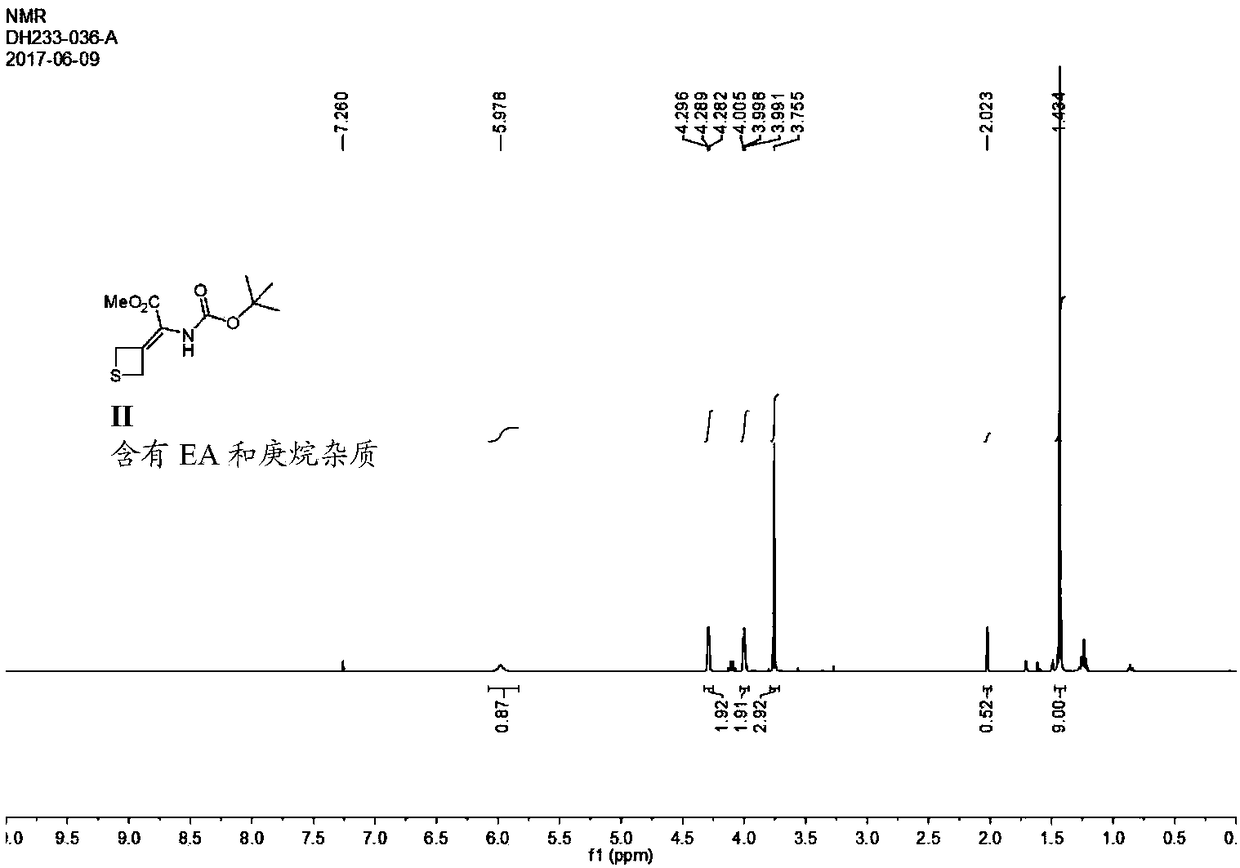
[0054] Synthesis of Embodiment 1 Compound II
Embodiment 1
[0056] Under stirring, 152 g of DBU was completely dissolved in 400 mL of dichloromethane to prepare an alkaline solution. And the alkali solution was cooled to 0°C in an ice-water bath for use.
[0057] 23.4 g of (±)-BOC-A-phosphonoglycine trimethyl ester was dissolved in 100 mL of dichloromethane, and then the above alkali solution was added dropwise at 0° C. to form a phosphorus ylide solution.
[0058] Next, 5.4 g of thietanone was dissolved in 100 mL of dichloromethane, and then, this solution was added dropwise to the above-mentioned phosphorus ylide solution. At this time, the reaction solution changed from yellow to brown, and then the reaction mixture was warmed to room temperature and reacted overnight. The progress of the reaction was monitored by TLC. After the reaction was completed, dilute hydrochloric acid was added to quench the reaction. The organic phase was obtained after standing for separation. Subsequently, the organic phase was successively washed wi...
Embodiment 2
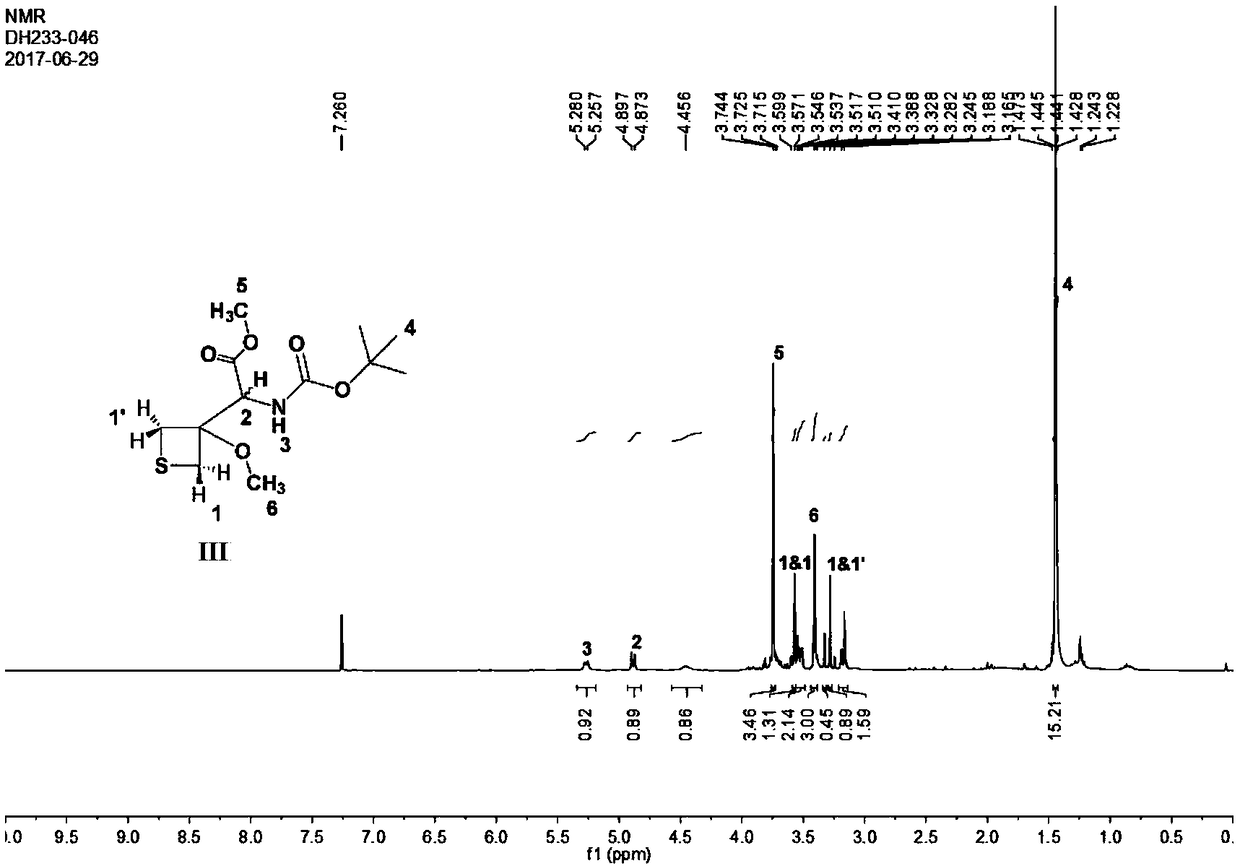
[0060] Example 2 was carried out in the same manner as in Example 1 above, except that the base used was triethylamine. The product was confirmed by nuclear magnetic resonance spectrum, and the yield was 23%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com