Application of resveratrol-loading human multipotential stem cell exosome in preparing drug for treating refractory skin wound related diseases
A technology of human pluripotent stem cells and resveratrol, applied in non-embryonic pluripotent stem cells, artificially induced pluripotent cells, skin diseases, etc., can solve the problems that the treatment potential of diseases and injuries has not been fully utilized and needs to be further confirmed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Culture of human embryonic stem cells (ESCs) and extraction and identification of exosomes
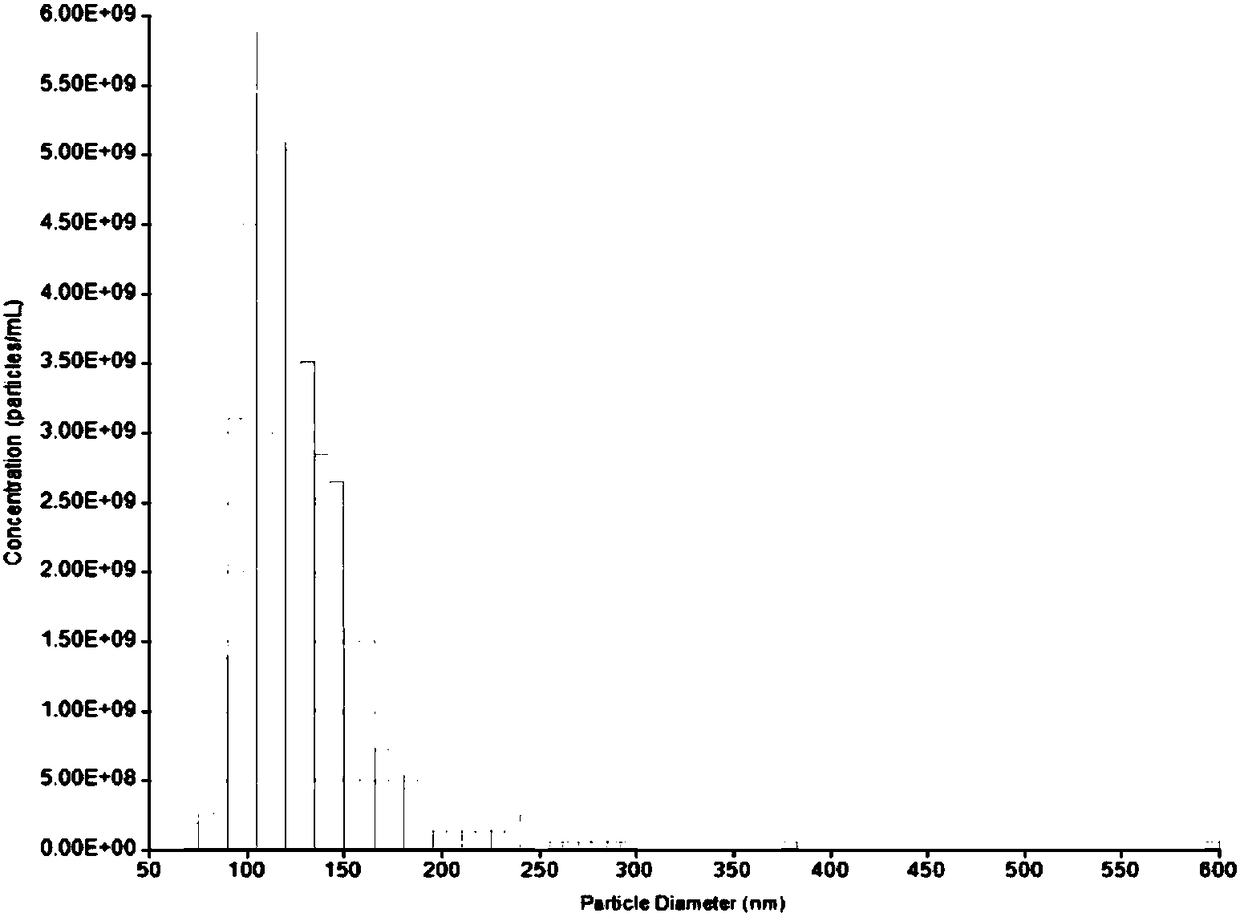
[0095] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), ESCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), in an incubator (37°C, 5% CO 2 , saturated humidity) culture, and collect the culture medium changed every day. Filter the medium through a 0.22 micron pore size filter membrane and centrifuge at 10,000g at 4°C for 30 minutes to remove cell debris; use a 100KD molecular weight ultrafiltration tube and centrifuge (3500g, 15min) to intercept exosomes in the concentrated supernatant to obtain exosomes concentrate; transfer the concentrate to a 30% sucrose / heavy water density pad (1.210 g / cm 3 ), centrifuge at 100,000g at 4°C for 210 minutes, collect the 5ml sucrose / heavy water density pad at the bottom, add PBS to dilute, transfer to an ultrafiltration centrifuge tube with a molecular weigh...
Embodiment 2
[0100] Culture of human induced pluripotent stem cells (iPSCs) and extraction and identification of exosomes
[0101] A layer of embryonic stem cell Matrigel (ESC-Qualified BD Matrigel, BD Sparks, MD, USA), iPSCs were moved into the dish, and mTeSR1 serum-free medium (StemCell Vancouver, BC, Canada), in an incubator (37°C, 5% CO 2 , saturated humidity) culture, and collect the culture medium changed every day. Filter the medium through a 0.22 micron pore size filter membrane and centrifuge at 10,000g at 4°C for 30 minutes to remove cell debris; use a 100KD molecular weight ultrafiltration tube and centrifuge (3500g, 15min) to intercept exosomes in the concentrated supernatant to obtain exosomes concentrate; transfer the concentrate to a 30% sucrose / heavy water density pad (1.210 g / cm 3 ), centrifuge at 100,000g at 4°C for 210 minutes, collect the 5ml sucrose / heavy water density pad at the bottom, add PBS to dilute, transfer to an ultrafiltration centrifuge tube with a mol...
Embodiment 3
[0106] Human ESC-derived exosomes (ES-exo) encapsulated resveratrol (Res) by co-incubation method
[0107] ES-exo solution is from Example 1.
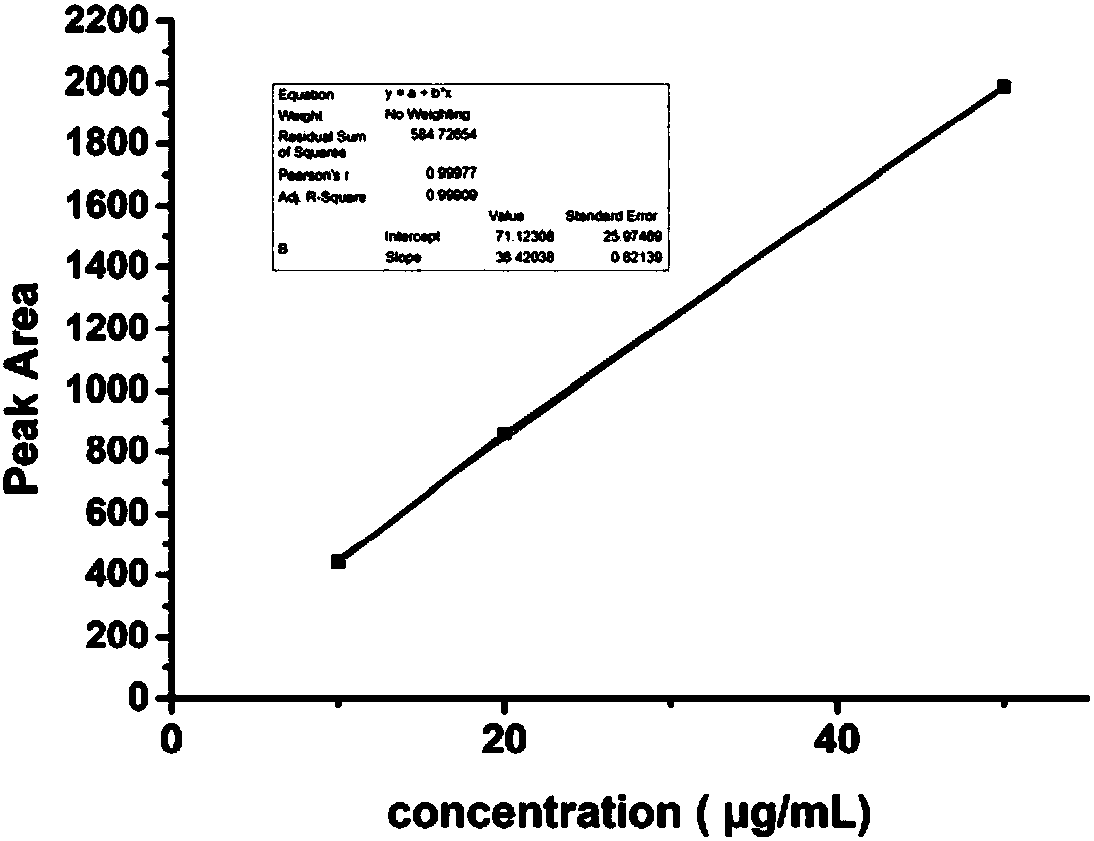
[0108] Quantitative standard curve determination of Res: Accurately weigh 1mg of Res powder and dissolve it in 1mL of methanol, then dilute the solution with methanol to prepare 50μg / mL, 20μg / mL, 10μg / mL Res in methanol standard solution, HPLC injection. The chromatographic conditions are as follows:
[0109] Chromatographic column: Zorbax Extend C-18, 150*4.6μm, 5–micro
[0110] Mobile phase: methanol: water = 95:5
[0111] Flow rate: 1mL / min
[0112] Column temperature: room temperature
[0113] Detection wavelength: 305nm
[0114] After obtaining the corresponding experimental results, the peak area (PA) of the chromatographic peak was plotted as a function of the Res concentration (C, μg / mL) (see attached image 3 ), obtain the quantitative standard curve of Res under this chromatographic separation condition as follows:
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com