Therapeutic use of a sterile aqueous ophthalmic solution
An ophthalmic solution, water-based technology, applied in the direction of organic active ingredients, inorganic inactive ingredients, medical preparations of inactive ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
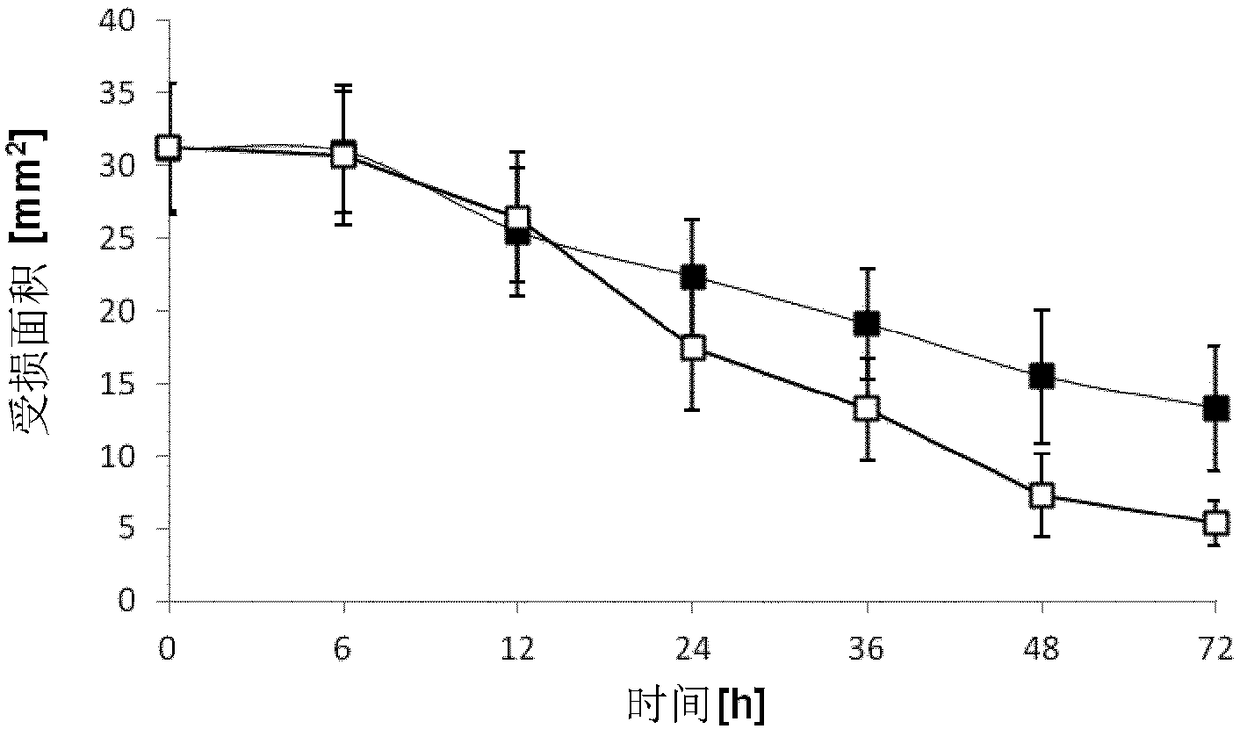
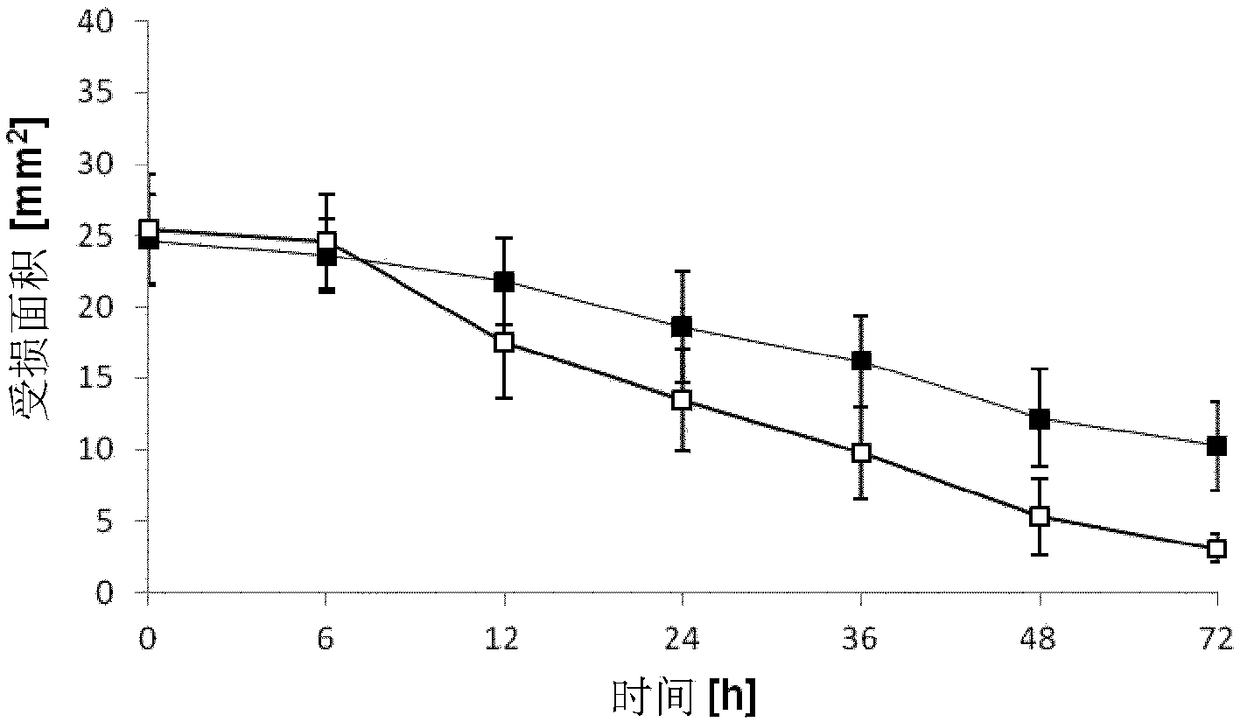
[0090] Example 1: Corneal Wound Healing Model
[0091] The purpose of this experiment was to evaluate the aqueous ophthalmic solution containing 0.1% chitosan-NAC (degree of modification: 209 μM free thiol groups / g polymer) compared to a control substance consisting of phosphate buffered saline (PBS). Whether the solution can speed up the wound healing process. Prepared under inert conditions containing 0.1% (w / w) Aqueous ophthalmic solution of chitosan-NAC. The final pH of the solution was 6.4. Female New Zealand White rabbits (Charles River, Ekrath, Germany) weighing 1.7 kg to 2.5 kg were used in this experiment. Animals were housed in pairs in a controlled environment: artificial circadian rhythm of 12 / 12 hours, room temperature 20°C, humidity 60%. Rabbits were provided with water and standard chow ad libitum. Animals were not started on studies until after a period of at least 2 weeks (to acclimate) after arrival.
[0092] A total of 16 animals were induced with de...
Embodiment 2
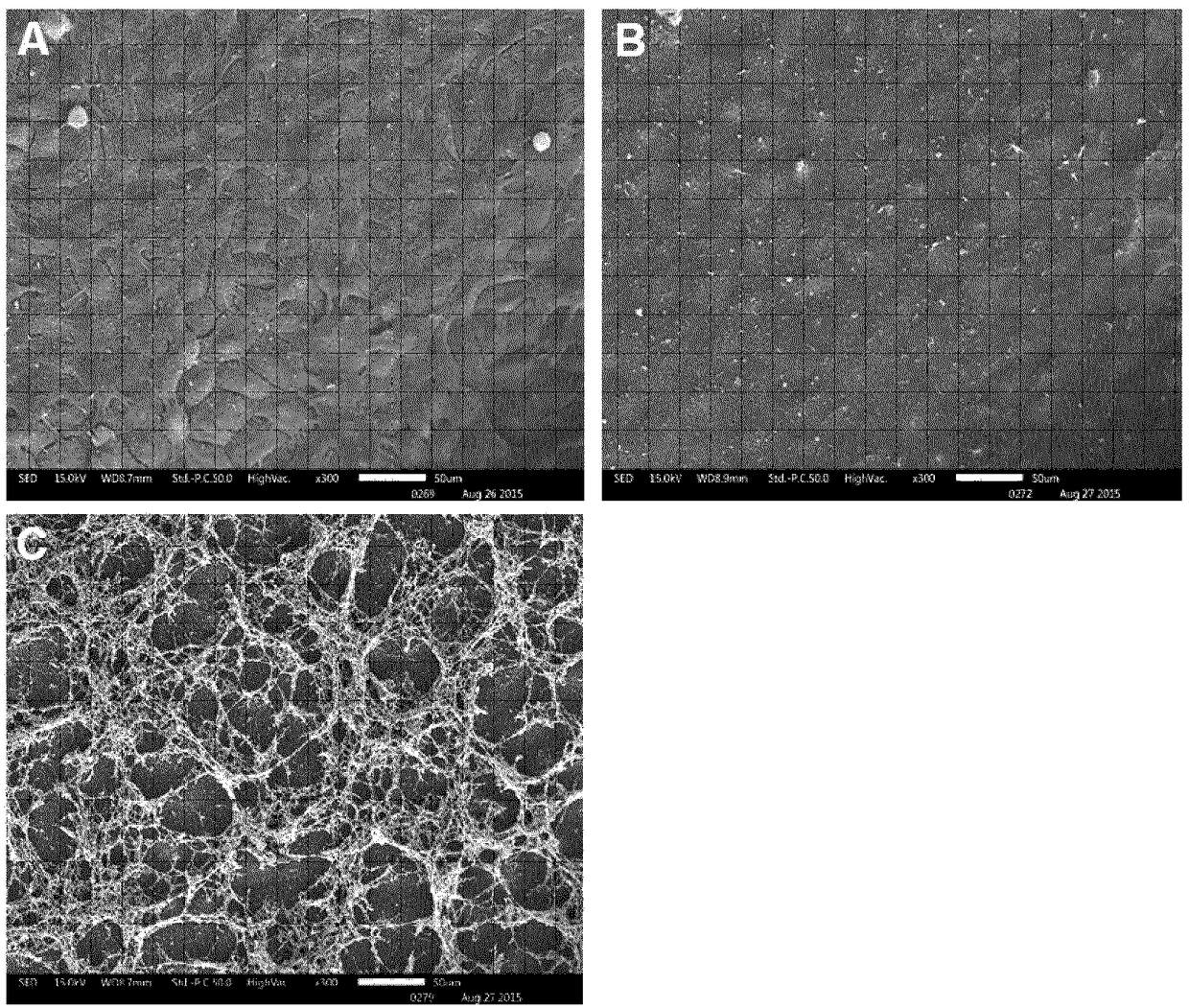
[0094] Example 2 The polymer network structure formed on the ocular surface tissue
[0095] Human limbal epithelial cells (HCLE) were incubated at 37°C and 5% CO 2 maintained in serum-free keratinocyte growth medium (Thermo Fisher Scientific), and seeded 1×10 5 cells / well into 24-well plates (equipped with 11 mm cover glass). When the cells reached confluence, they were treated with 0.1% (w / w) chitosan-HCl or 0.1% (w / w) chitosan-NAC (modification degree: 219 μM free thiol groups) diluted in 100 mM borate buffer. pellet / gram of polymer) for 15 minutes. Simultaneously, borate buffer was applied to control cells. Next, the polymer solution was removed and the cells were immediately fixed for scanning electron microscopy (SEM) by adding 2.5% glutaraldehyde in 0.06×PHEM buffer (1×PHEM buffer contained 60 mM PIPES, 25 mM HEPES, 10 mM EGTA and 2 mM MgCl 2 ) for cell fixation. Then, the monolayers were post-fixed in 1% aqueous osmium tetroxide (Agar Scientific, Stansted, UK),...
Embodiment 3
[0097] Example 3 Case Study
[0098] A 57-year-old male patient with extremely severe atopic dermatitis and multiple recurrent herpetic keratitis required urgent penetrating keratoplasty due to perforation. He subsequently developed a post-transplant infection requiring antibiotic control. However, he also suffered from a persistent epithelial defect that did not heal after several months despite several different medications (topical medications: Moxifloxacin (Vigamox) 4× / day, Dexa EDO 2× / day and Vismed once an hour; oral medication: valacyclovir (Valtrex) 1-0-1, mycophenolic acid extended-release tablets (Myfortic) 360mg 2-0-2, deoxycorticosterone (Decortin) H 12, 5mg / day).
[0099] After additional application of an aqueous ophthalmic solution containing 0.1% chitosan-NAC (modification degree: 212 μM free thiol groups / g polymer) to the eye (once a day for 7 days), the corneal epithelium Defects are cured.
[0100] Example 4 Case Study
[0101] A 31-year-old male co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com