A kind of recombinant protein and its preparation method and application
A recombinant protein and protein technology, applied in biochemical equipment and methods, chemical instruments and methods, recombinant DNA technology, etc., can solve the problems of connection ratio, difficult control of the molecular size of the connection site, and obstacles to drug quality control, etc., to improve Memory and cognition levels, reduced activation levels, simple effects of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
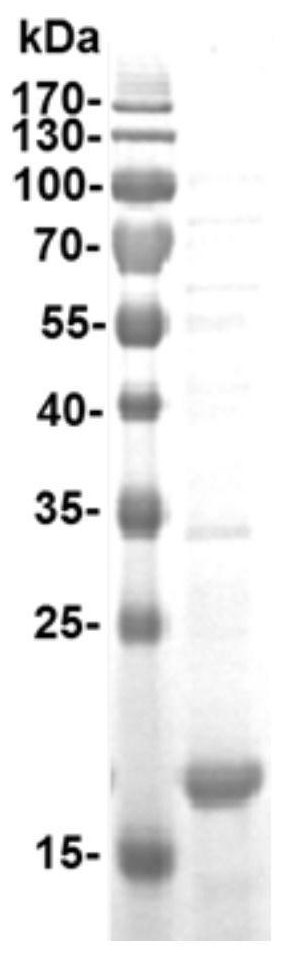
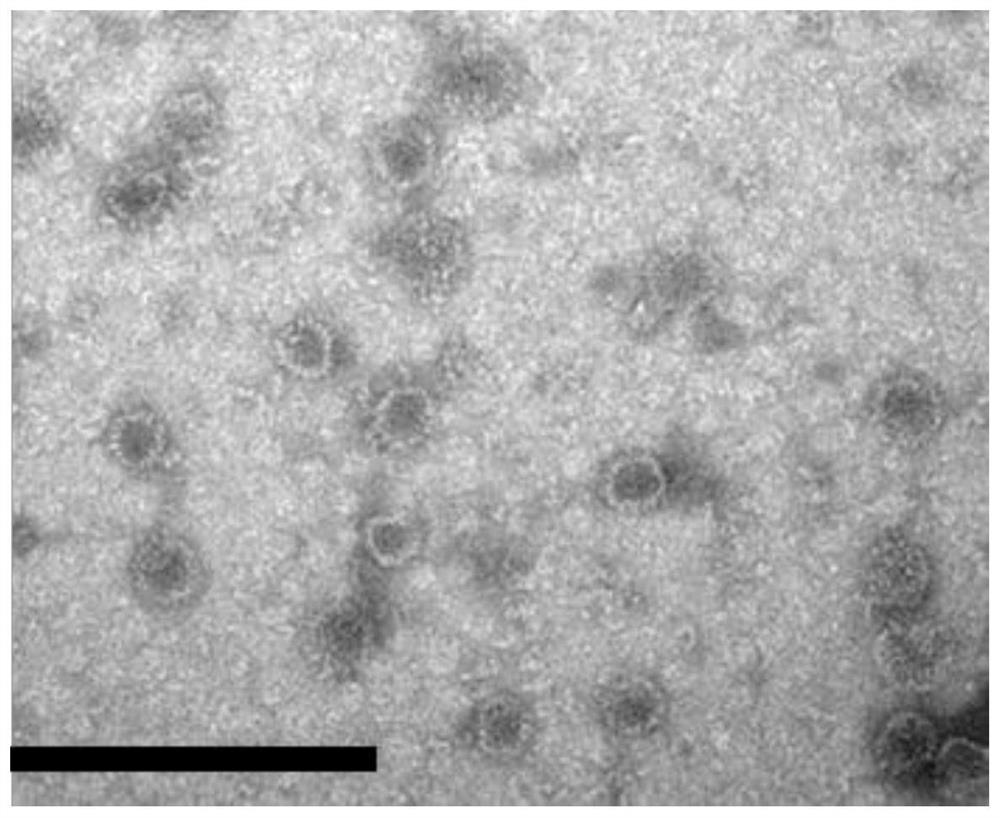
[0067] Example 1 Preparation and Characterization of Recombinant Proteins
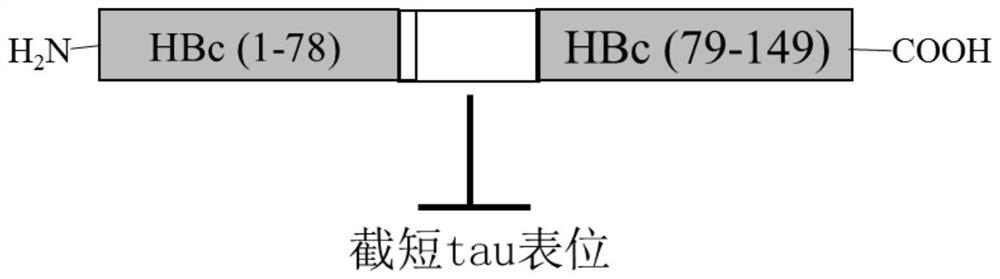
[0068] Will Tau 294-305 The gene is inserted between the codon sites of Asp78 and Pro79 in the immunodominant region of the truncated hepatitis B virus core protein (HBc) gene (coding amino acids 1 to 149 on HBc) to obtain T294-HBc recombinant protein; T294 will be expressed -The nucleic acid sequence of the HBc recombinant protein is cloned into the PBR327 vector to obtain the T294-HBc-PBR recombinant vector; the T294-HBc-PBR recombinant vector is transformed into Escherichia coli BL21 competent cells, and the Escherichia coli cells are supplemented with 1% casamino acids, Culture in M9 salt medium with 0.2% glucose and 50 μg / mL ampicillin at 37° C. for 20-24 hours.
[0069]Collect the cells by centrifugation, resuspend 2 g of cells in 40 mL of lysis buffer, and sonicate in an ice bath for 30 minutes; then, centrifuge at 12,000 rpm for 15 minutes, add ammonium sulfate to the supernatant to 33% satura...
Embodiment 2
[0073] Embodiment 2 Vaccine immunity and antibody detection
[0074] (1) Animal immunity
[0075] The 5-month-old Tau.P301S transgenic mice were randomly divided into PBS group, HBc control group, R1 group, R2 group, R3 group, R4 group and T294-HBc group, with 7 mice in each group. The same wild-type C57BL / 6J mice served as the WT control group.
[0076] The above 8 groups of mice were treated as follows:
[0077] Inject 100 μg of recombinant protein + aluminum adjuvant to mice in R1 group, R2 group, R3 group, R4 group and T294-HBc group, inject the same dose of PBS + aluminum adjuvant to mice in PBS group, and inject 100 μg to mice in HBc control group Equal doses of HBc carrier + aluminum adjuvant were injected into WT control mice with equal doses of aluminum adjuvant, and the mice were subcutaneously injected on days 0, 14, 28 and 42, with an interval of two weeks between each injection.
[0078] (2) Antibody titer and EC 50 detection
[0079] On the 10th day after ea...
Embodiment 3
[0092] Example 3 T294-HBc vaccine improves the cognitive level of Tau.P301S mice
[0093] In order to evaluate the therapeutic effect of T294-HBc vaccine on Tau.P301S mice, the mice after immunotherapy were subjected to the following behavioral experiments: forced Y maze, novel object recognition, water maze and spontaneous Y maze.
[0094] (1) Forced Y maze
[0095] The Y maze used in this experiment is composed of three arms with an angle of 120 degrees to each other. The end of each arm is marked with a different pattern. The three arms are randomly designated as the start arm, the new detection arm and the exploration arm.
[0096] In the training phase, put the mouse into the starting arm and let it explore freely in the starting arm and the exploration arm for 5 minutes. At this time, the new arm is closed; after 30 minutes, open the new arm and put the mouse in the Initiate arm and allow mice to explore freely for 5 min. The time each mouse stayed in the new arm and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com