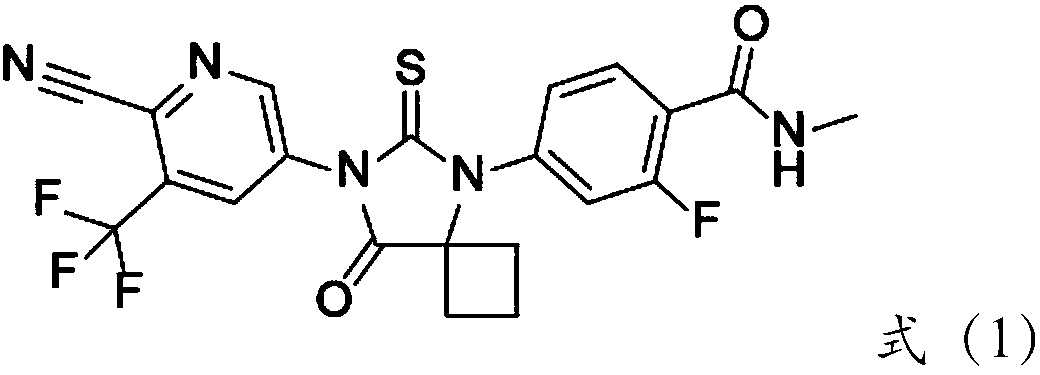
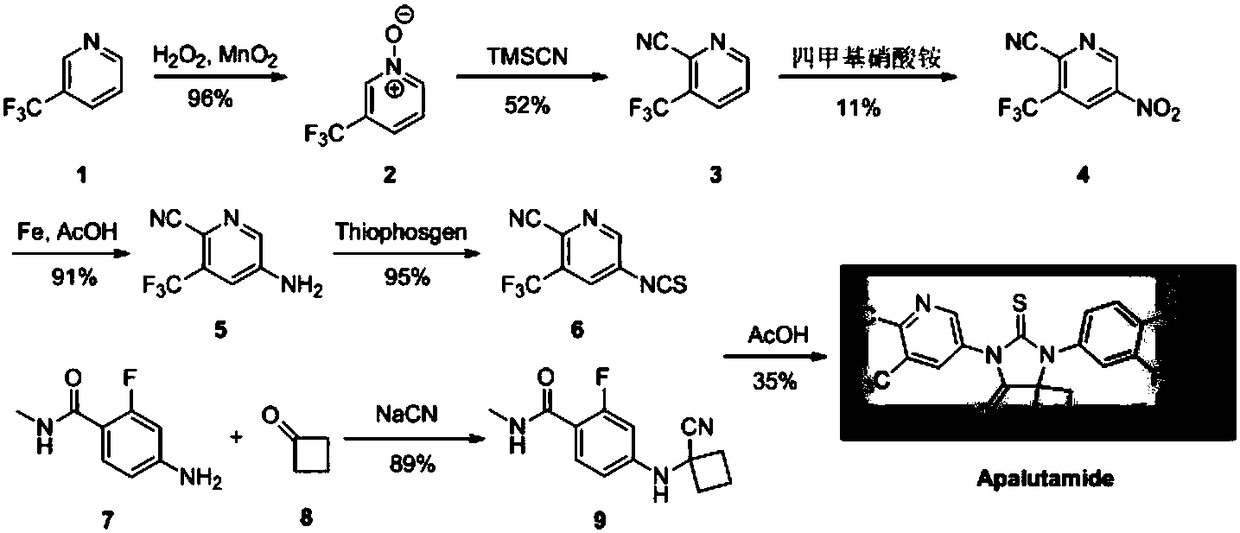
Method for using high-dispersion double-metal nano material to prepare drug intermediate for urogenital system
A highly dispersed and versatile technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of low yield, high production cost, unsuitable catalyst recovery, etc., and achieve high yield , post-processing is simple, easy to remove the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
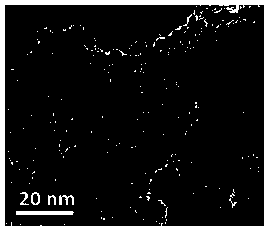
Image
Examples
Embodiment 1
[0040] The preparation method of the two-component catalyst of supported type highly dispersed Pd / Ni co-doped, comprises the following steps:
[0041] 1) Dissolve 10mmol of 2-aminoterephthalic acid in potassium hydroxide ethanol aqueous solution with pH=8-9, ultrasonically dissolve, add 10mmol of nickel chloride hexahydrate into the system, and then transfer to the polytetrafluoroethylene-lined In a stainless steel reaction kettle, conduct hydrothermal treatment at 150-180°C for 8-12h in a sealed environment, cool down to room temperature naturally, filter, then place in 20ml of absolute ethanol, beat at 40-60°C for 1-2h, and then filter, 50-60 Vacuum drying at ℃ to obtain nickel 2-aminoterephthalate;
[0042] 2) Disperse 100.0 g of nickel 2-aminoterephthalate in 500 ml of deionized water, then adjust the pH of the system to 3.0-3.8 with 1 mol / L hydrochloric acid aqueous solution, and slowly add 40 ml of 2 mol / L potassium tetrachloropalladate aqueous solution dropwise , keep ...
Embodiment 2
[0044] Catalyst performance evaluation, the reaction conditions are as follows:
[0045] Add 2-bromo-5-nitro-3-(trifluoromethyl)pyridine (2.70g, 10mmol, HPLC purity is 99.8%) in 50ml three-necked flask, 10ml solvent NMP, cyanation reagent CuCN 15mmol, catalyst 0.30g 120 DEG C of reaction, when HPLC monitors that the substrate is no longer converted, the reaction is stopped, and the substrate conversion rate and product selectivity of each reaction liquid system are counted. The results are shown in Table 1:
[0046] Table 1 Different catalyst reaction results
[0047] Catalyst type
Conversion rates / %
selectivity / %
Embodiment 1 preparation
89.8
93.1
Pd / C (5%wtPd content)
78.2
96.3
Pd(OAc) 2
65.2
94.3
Pd(PPh 3 ) 4
46.3
—
PD 2 (dba) 3
59.3
—
NA
69.8
91.4
[0048] Note: NA means that no catalyst was added, and "—" means that the selectivity data was not counte...
Embodiment 3
[0051] For the screening of solvent types and cyanation reagents, the reaction conditions are as follows:
[0052] Add 2-bromo-5-nitro-3-(trifluoromethyl)pyridine (2.70g, 10mmol, HPLC purity is 99.8%) in the 50ml three-necked flask, 10ml solvent, cyanation reagent 15mmol, catalyst (embodiment 1 Preparation) 0.30g 120 DEG C of reaction, when HPLC monitoring substrate no longer converts, stop reaction, count the substrate conversion rate and the selectivity of product of each reaction liquid system, the result is as shown in table 2:
[0053] Table 2 The influence of different solvents and cyanation reagents on the reaction
[0054]
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com