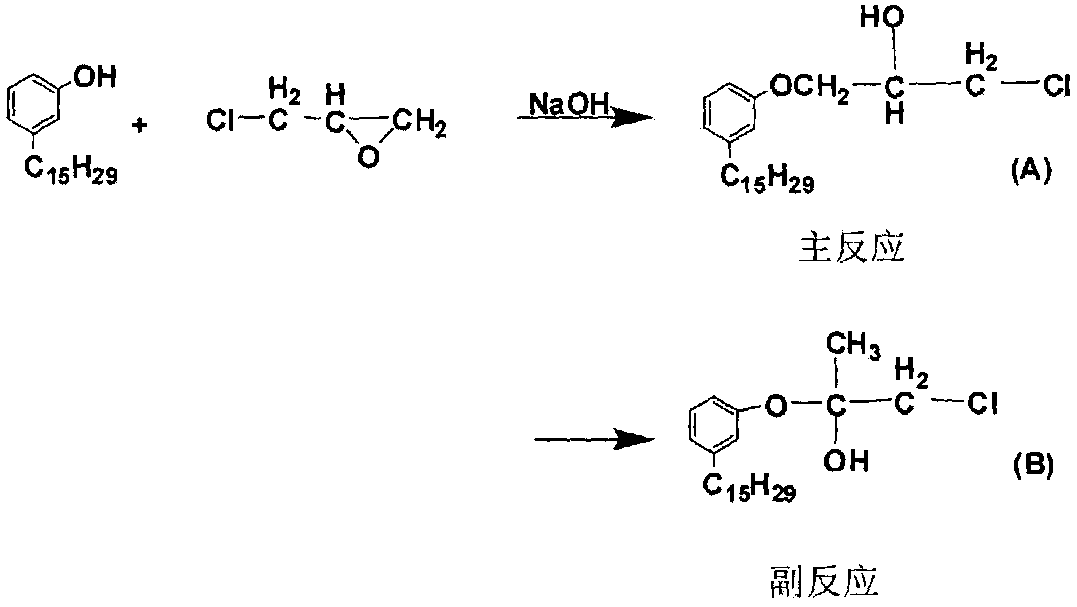
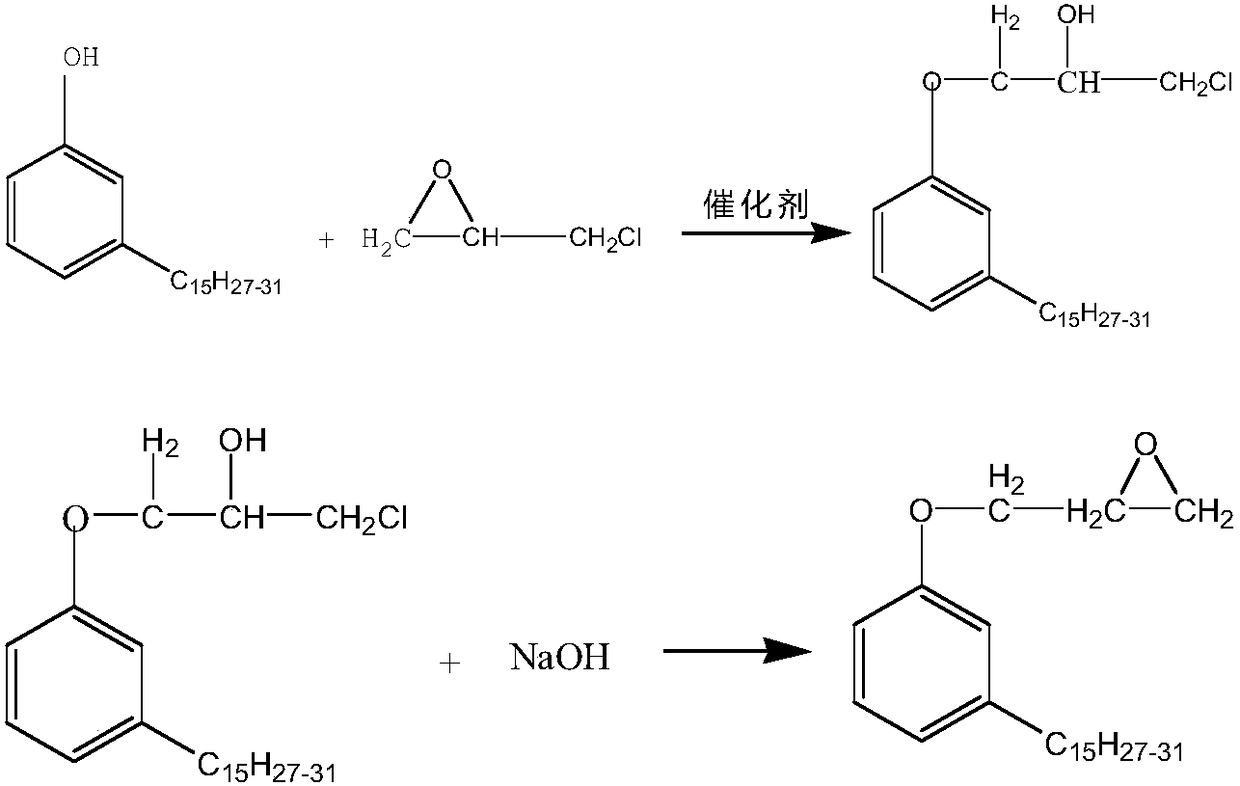
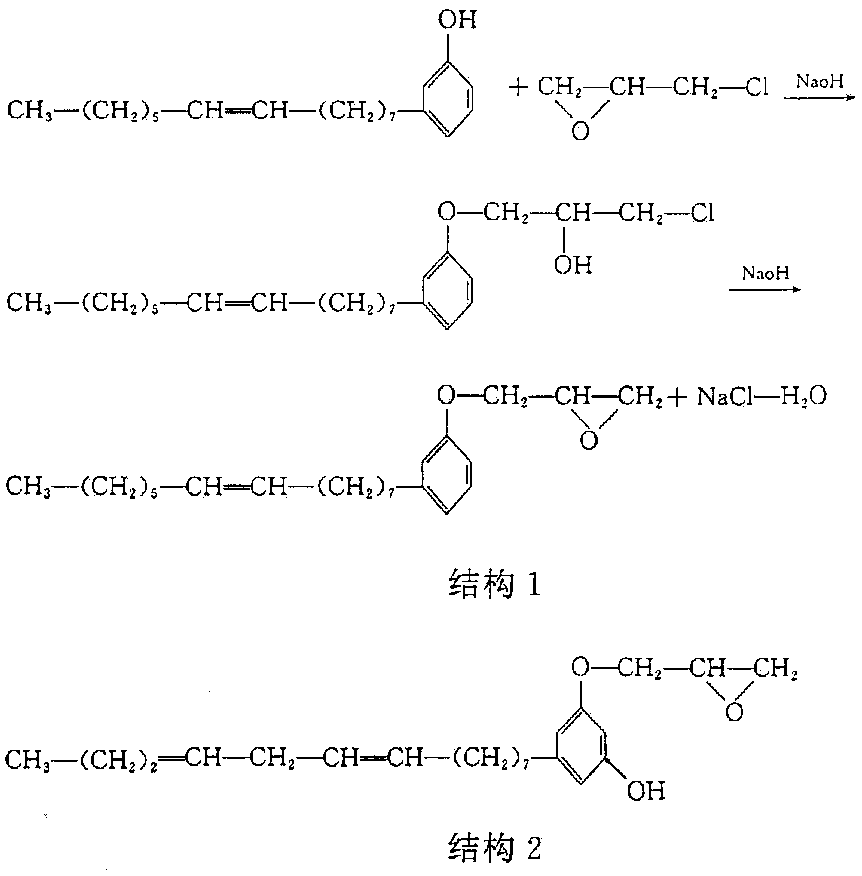Preparation method of anacardol glycidyl ether
A technology of glycidyl ether and cardanol, which is applied in the chemical industry, can solve the problems of large amount of epichlorohydrin, dark reaction color, and long reaction time, and achieve low hydrolysis chlorine, low epoxy equivalent, and reduction of free cardanol The effect of content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the preparation method of cardanol glycidyl ether
[0048] 1. Raw materials: cardanol, epichlorohydrin, sodium hydroxide, the molar ratio of the three is 1:3.6:1.3, the catalyst (tetraethylammonium bromide and tetramethylammonium bromide is 5: 1 mixed mixture), the dosage is 1‰ of the quality of cardanol.
[0049] 2. Preparation method:
[0050] 1) Take cardanol, epichlorohydrin, sodium hydroxide and catalyst according to the proportioning ratio, and make sodium hydroxide into a 40% sodium hydroxide solution for subsequent use;
[0051] 2) Mix cardanol, epichlorohydrin and catalyst, stir and heat up to 50°C, and slowly add sodium hydroxide solution with a concentration of 40% after constant temperature (the molar ratio of cardanol to sodium hydroxide is 1:1.1, accounting for 84.6% of the total moles of sodium hydroxide), after stirring at a constant temperature of 50-70°C for 0.5 hours, the dripping method of constant temperature (68°C) vacuum distillati...
experiment example 1
[0056] 1. Sample:
[0057] Sample 1 is embodiment 1;
[0058] Reference substance 1: adopt existing technology such as the scheme and preparation method in "Preparation of Cardanol Glycidyl Ether" by Xu Li et al. (Xu Li et al., Preparation of Cardanol Glycidyl Ether, Fine Petrochemical Industry, Vol. Phase 1 January 2013: 1-4), the proportion of this document is that the molar ratio of cardanol, epichlorohydrin and sodium hydroxide is 1:6:3, the amount of catalyst is 2%, and the four ingredients are added In a three-necked flask, react at a constant temperature of 70°C for 10 hours, cool to room temperature, add petroleum ether to wash and filter to remove salt, wash with water to neutrality, remove unreacted epichlorohydrin and solvent by rotary evaporation, and finally obtain cardanol glycidyl ether . The yield is as high as 89.1%;
[0059] Reference substance 2: replace the catalyzer among the embodiment 1 with tetrabutyl ammonium bromide, consumption is identical with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com