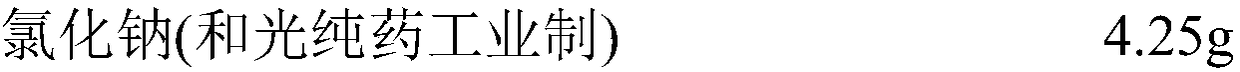Dried influenza vaccine preparation and method for producing dried influenza vaccine preparation
A flu vaccine, drying technology, applied in antiviral agents, freeze-dried delivery, pharmaceutical formulations, etc., can solve the problem of difficult storage of multiple flu HA vaccines, and achieve the effect of stable supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~40
[0084] (freeze-dried influenza HA vaccine preparation)
[0085] For influenza HA antigens (A H1N1: A / California / 7 / 2009, A H3N2: A / Victoria / 361 / 2011, B Yamagata system: B / Wisconsin / 1 / 2010 and B Victoria system: B / Brisbane / 60 / 2008, manufactured by the Osaka University Microbial Disease Research Association), as shown in Tables 1 to 4, trehalose (manufactured by Hayashibara) or sucrose (manufactured by Wako Pure Chemical Industries) was added as a disaccharide, and L(+ )-arginine hydrochloride (manufactured by Wako Pure Chemical Industries), L-lysine hydrochloride (manufactured by Wako Pure Chemical Industries), L(-)-proline (manufactured by Wako Pure Chemical Industries), L- After threonine (manufactured by Wako Pure Chemical Industries) or L(+)-arginine (manufactured by Wako Pure Chemical Industries), PBS (phosphate-buffered sodium chloride solution) with the following composition was added, and the above-mentioned disaccharide The content is 10% by mass / volume, the content o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com