Method for producing N-acetylneuraminic acid through recombinant bacillus subtilis whole-cell transformation
A technology of Bacillus subtilis and neuraminidase aldolase, which is applied in the field of genetic engineering, can solve the problems of high cost, difficult separation, complicated process and the like, and achieves the effects of simple method, good application prospect and easy use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Construction of recombinant plasmid
[0034] Primers were designed according to the N-acetylneuraminic acid aldolase gene (NanA) of Escherichia coli (Escherichia coli K12, GenBank: U05248.1) published on NCBI:
[0035] NanA-F: '-CGGGGTACCATTATAGGTAAGAGAGGAATGTACACATGGCAACGAATTTACGTGGCG3', NanA-R: 5'-TTGCCCATTGATCCTTCCTCCTTTTCACCCGCGCTCTTGCATC-3', using the synthetic N-acetylneuraminic acid synthase group as a template to amplify the gene NanA fragment, according to the NCBI published on the Candida (Anabaena sp. .CH1, GenBank: DQ661858.1) N-acetylglucosamine isomerase gene (AGE), after optimized codon preference by Bacillus subtilis, gene synthesis (as shown in SEQ ID NO.4), design primers:
[0036]AGE-F: 5'-GATGCAAGAGGCGCGGGTGAAAAGGAGGAAGGATCAATGGGCAAAAACTTACAAGCTCTGG-3', AGE-R: 5'-TCCCCCGGGTTATGAAAGTGCTTCAAACTGTTGCC-3',
[0037] Use the above primers to amplify the AGE gene fragment with the synthetic N-acetylglucosamine isomerase base as the template, the ...
Embodiment 2
[0038] Example 2 Construction of recombinant pP43NMK-NA plasmid Bacillus subtilis
[0039] The constructed pP43NMK-NA plasmid was transformed into Bacillus subtilis B6CG. Using NanA-F and AGE-R primers to select transformants for colony PCR, the gel electrophoresis confirmed that a 2115bp band appeared, which confirmed that the recombinant Bacillus subtilis B6CGNA was successfully constructed.
Embodiment 3
[0040] Example 3 Whole cell transformation method to produce N-acetylneuraminic acid
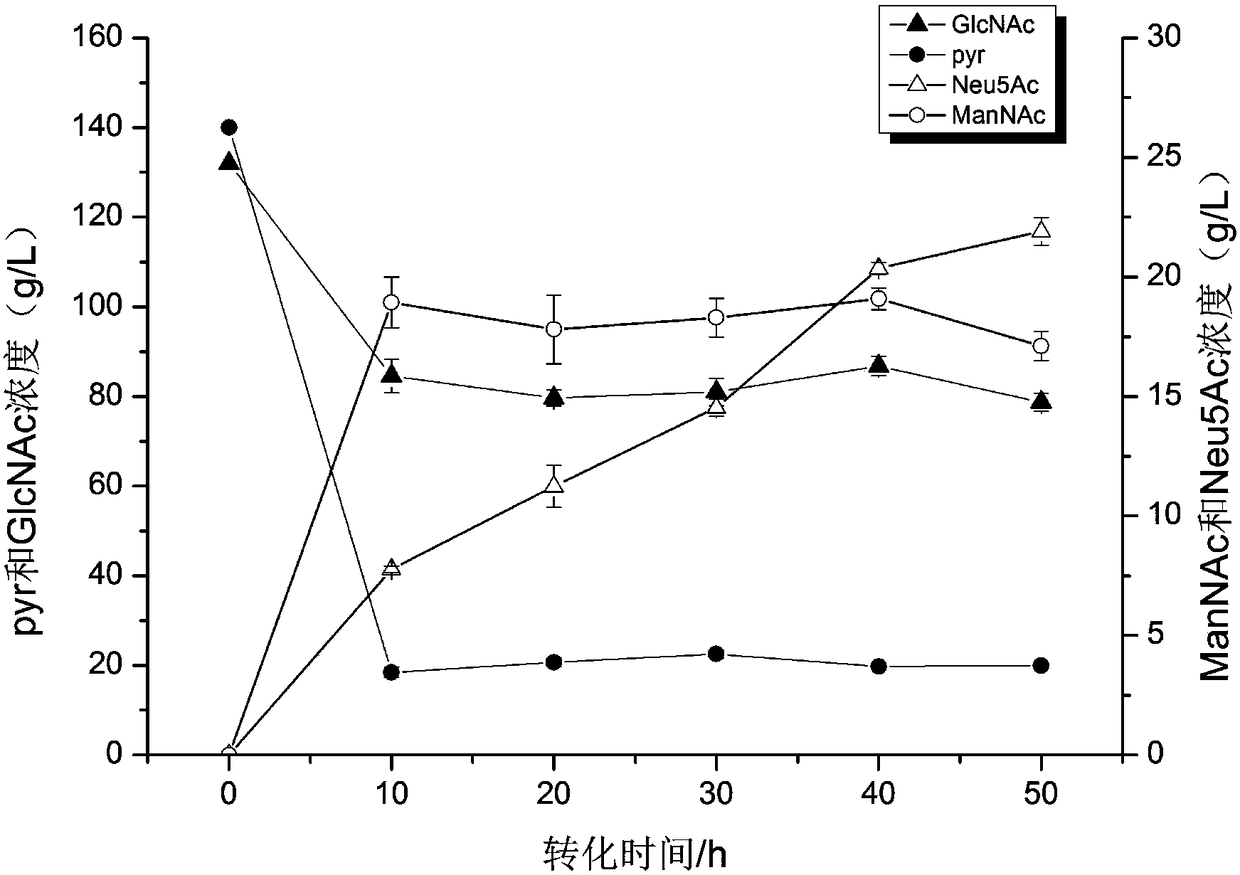
[0041] Collect the recombinant Bacillus subtilis B6CGNA fermented at 37°C and 200rpm for 22h and add 0.01M, PBS, 1.6M sodium pyruvate according to the bacterial addition amount of 15~20OD / mol sodium pyruvate and 30~40OD / mol N-acetylglucosamine , 0.6MN-acetylglucosamine, 0.01M MgCl 2 , 0.4% TritonX-100 whole cell transformation system, OD kept at 30, pH 7.2 ~ 7.4. 30 ℃ 220rpm, sampling at intervals from 0h to 60h, centrifugation to take the supernatant to detect N-acetylneuraminic acid production, detection The yield was 21.89g / L at 50h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com