Preparation method of cobalt vanadium duplex-metal hydroxide nanosheet as catalyst in electrolysis of water for oxygen evolution
A technology of hydroxides and nanosheets, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of high energy consumption, high risk, and high temperature. Achieve the effects of no post-processing, low synthesis temperature, and excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
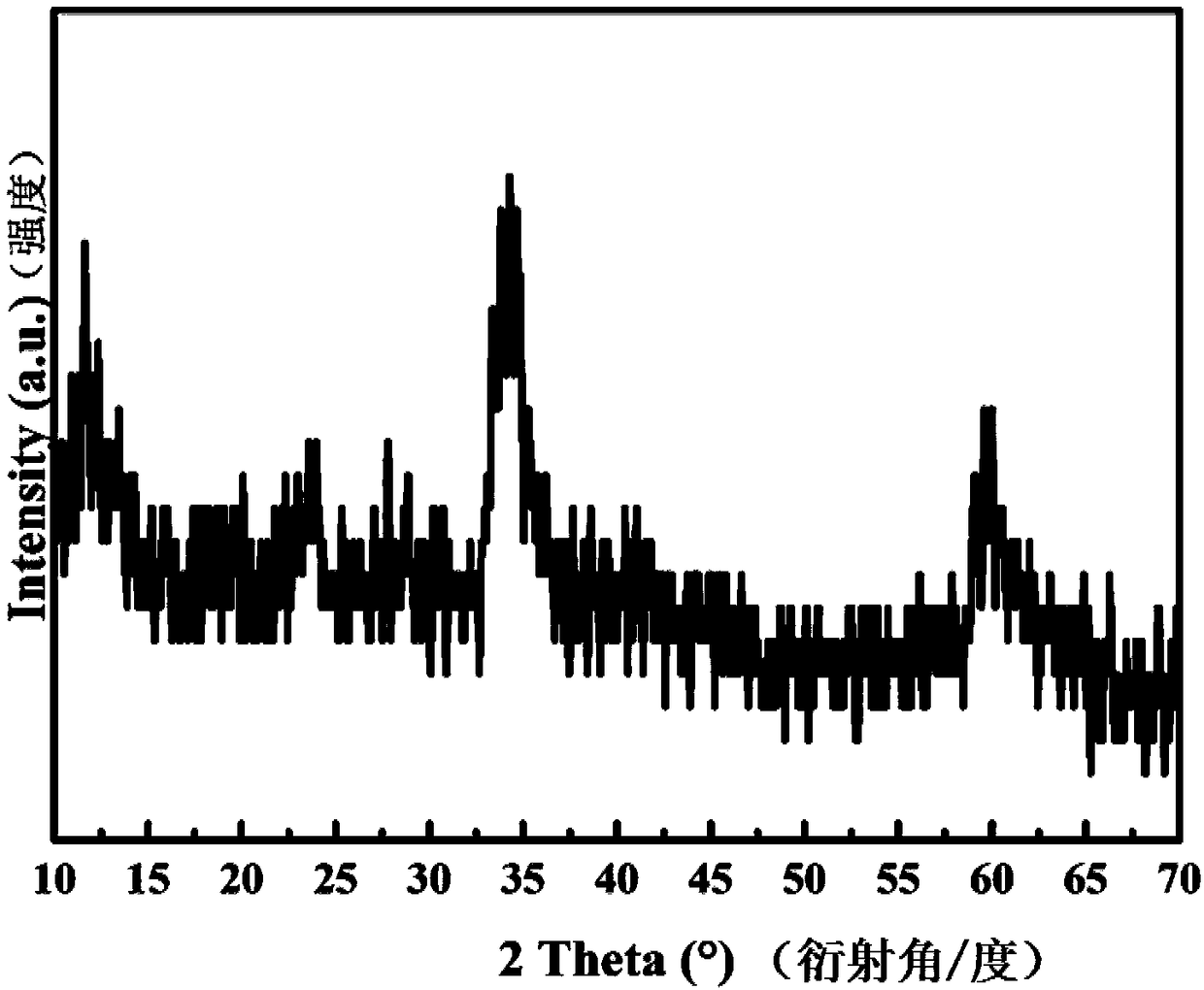
Image
Examples
Embodiment 1
[0022] 1) Take cobalt chloride hexahydrate, vanadium chloride and hexamethylenetetramine simultaneously and add them to 30ml of deionized water at room temperature under magnetic stirring to obtain a clear solution A with a cobalt salt concentration of 0.0426mol / L;
[0023] The molar ratio of cobalt chloride hexahydrate: vanadium chloride: hexamethylenetetramine is 1.28:0.32:2;
[0024] 2) Pour the clear solution A of step 1) into the reaction lining at a filling ratio of 60% and then seal it, then put the lining in an outer kettle and place it in a homogeneous reactor for 12 hours at 120°C;
[0025] 3) After the hydrothermal reaction is completed, the reaction kettle is naturally cooled to room temperature, and the reacted product is washed alternately with water and alcohol three times, then the product is collected and vacuum dried at 35° C. for 10 hours to obtain CoV-LDH nanosheets.
Embodiment 2
[0027] 1) Take cobalt chloride hexahydrate, vanadium chloride and hexamethylenetetramine simultaneously and add them to 25ml of deionized water at room temperature under magnetic stirring to obtain a clear solution A with a cobalt salt concentration of 0.0276mol / L;
[0028] The molar ratio of cobalt chloride hexahydrate: vanadium chloride: hexamethylenetetramine is 1.2:0.4:0.5;
[0029] 2) Pour the clear solution A from step 1) into the reaction lining at a filling ratio of 20% and then seal it, then put the lining in an outer kettle and place it in a homogeneous reactor for 24 hours at 80°C;
[0030] 3) After the hydrothermal reaction is completed, the reaction kettle is naturally cooled to room temperature, and the reacted product is washed alternately with water and alcohol three times, then the product is collected and vacuum dried at 25° C. for 12 hours to obtain CoV-LDH nanosheets.
Embodiment 3
[0032] 1) Take cobalt chloride hexahydrate, vanadium chloride and hexamethylenetetramine simultaneously and add them to 20ml of deionized water under magnetic stirring at room temperature to obtain a clear solution A with a cobalt salt concentration of 0.053mol / L;
[0033] The molar ratio of cobalt chloride hexahydrate: vanadium chloride: hexamethylenetetramine is 1.067:0.53:1.5;
[0034] 2) Pour the clear solution A of step 1) into the reaction liner at a filling ratio of 40% and then seal it, then put the liner in an outer kettle and place it in a homogeneous reactor for 10 hours at 150°C;
[0035] 3) After the hydrothermal reaction is completed, the reaction kettle is naturally cooled to room temperature, and the reacted product is washed alternately with water and alcohol three times, then the product is collected and vacuum dried at 30° C. for 12 hours to obtain CoV-LDH nanosheets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com