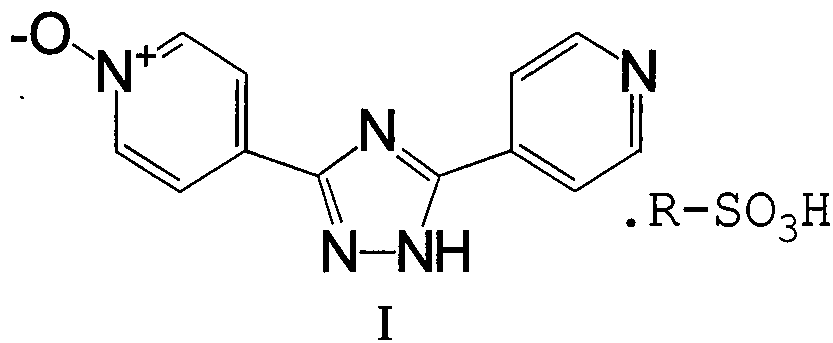
Intermediate for topipitastat, preparation method thereof, and method for preparing topipitastat from intermediate
A technology of topicastat and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of residual by-products, high cost, complicated procedures, etc., and achieve the effects of reducing the generation of three wastes, reducing working hours, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Preparation of isonicotinic acid formyl hydrazide nitrogen oxide
[0048] Put 153g of methyl isonicotinate nitrogen oxide, 100g of hydrazine hydrate (85%), and 1.5L of methanol into a 3L single-necked flask, heat up to 50°C while stirring, react for 8h, cool down to below 20°C, filter, and use the filter cake After washing with 300ml of cold methanol, it was dried at 55°C for 8h to obtain 120g of off-white solid with a yield of 78.4%.
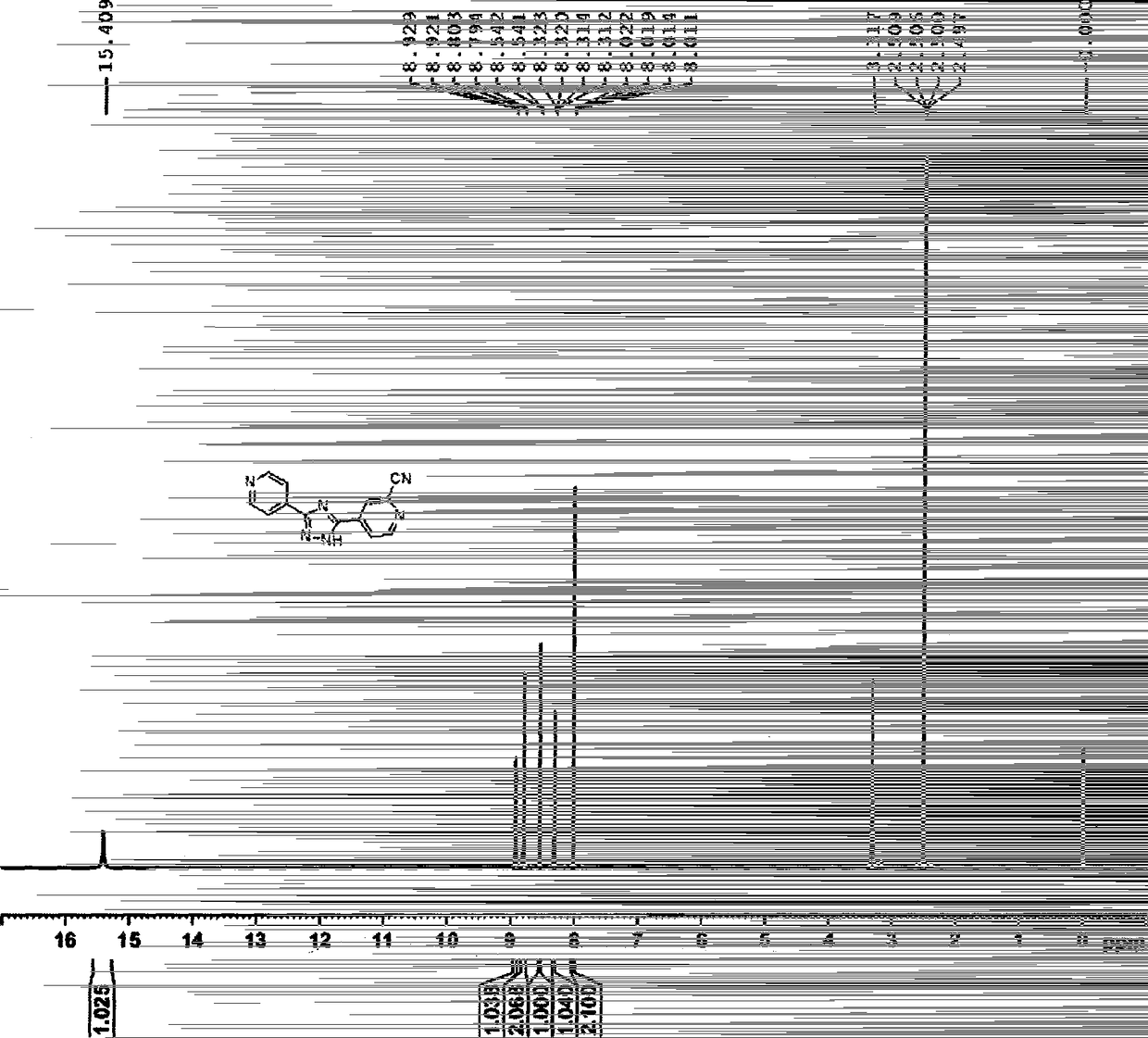
[0049] 2) Preparation of 3-(4-pyridyl)-5-(1-oxo-4-pyridyl)-1,2,4-triazole
[0050] Take 115g of 4-cyanopyridine, dissolve it in 1150ml of methanol, add 30g of sodium methoxide, stir at room temperature for 1 hour, add 150g of isonicotinic acid formohydrazide, raise the temperature to 90°C, all the solids are dissolved, and continue to reflux to gradually precipitate a light yellow solid. After 20 hours of reaction, the temperature was lowered to below 20°C, 1150ml of acetonitrile was added, stirred for 1h, the solid was filtered out,...
Embodiment 2
[0058] 1) Preparation of isonicotinic acid formyl hydrazide nitrogen oxide
[0059] Put 3000g of methyl isonicotinate nitrogen oxide, 2000g of hydrazine hydrate (85%), and 30L of methanol into a 50L glass jacketed reaction kettle, heat up to 50°C under stirring, react for 8h, cool down to below 20°C, filter, filter After the cake was washed with 6000ml of cold methanol, it was vacuum-dried at 55°C for 12h to obtain 2870g of off-white solid with a yield of 95.6%.
[0060] 2) Preparation of 3-(4-pyridyl)-5-(1-oxo-4-pyridyl)-1,2,4-triazole
[0061] Take 2850g of 4-cyanopyridine, 30L of methanol, and 450g of sodium methoxide, put them into a 100L glass jacketed reaction kettle, stir at room temperature for 1 hour, add 3370g of isonicotinic acid formohydrazide, raise the temperature to 90°C, all the solids are dissolved, and continue to reflux A light yellow solid gradually precipitated, and after 20 hours of reaction, the temperature was lowered to below 20°C, 30L of acetonitrile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com