Ethambutol hydrochloride synthesis method
A technology of ethambutol hydrochloride and butanol, which is applied in the directions of organic chemistry methods, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of many synthesis procedures, complicated operations, large equipment corrosion, etc., and shortens the technological process. , The effect of simplifying the synthesis process and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
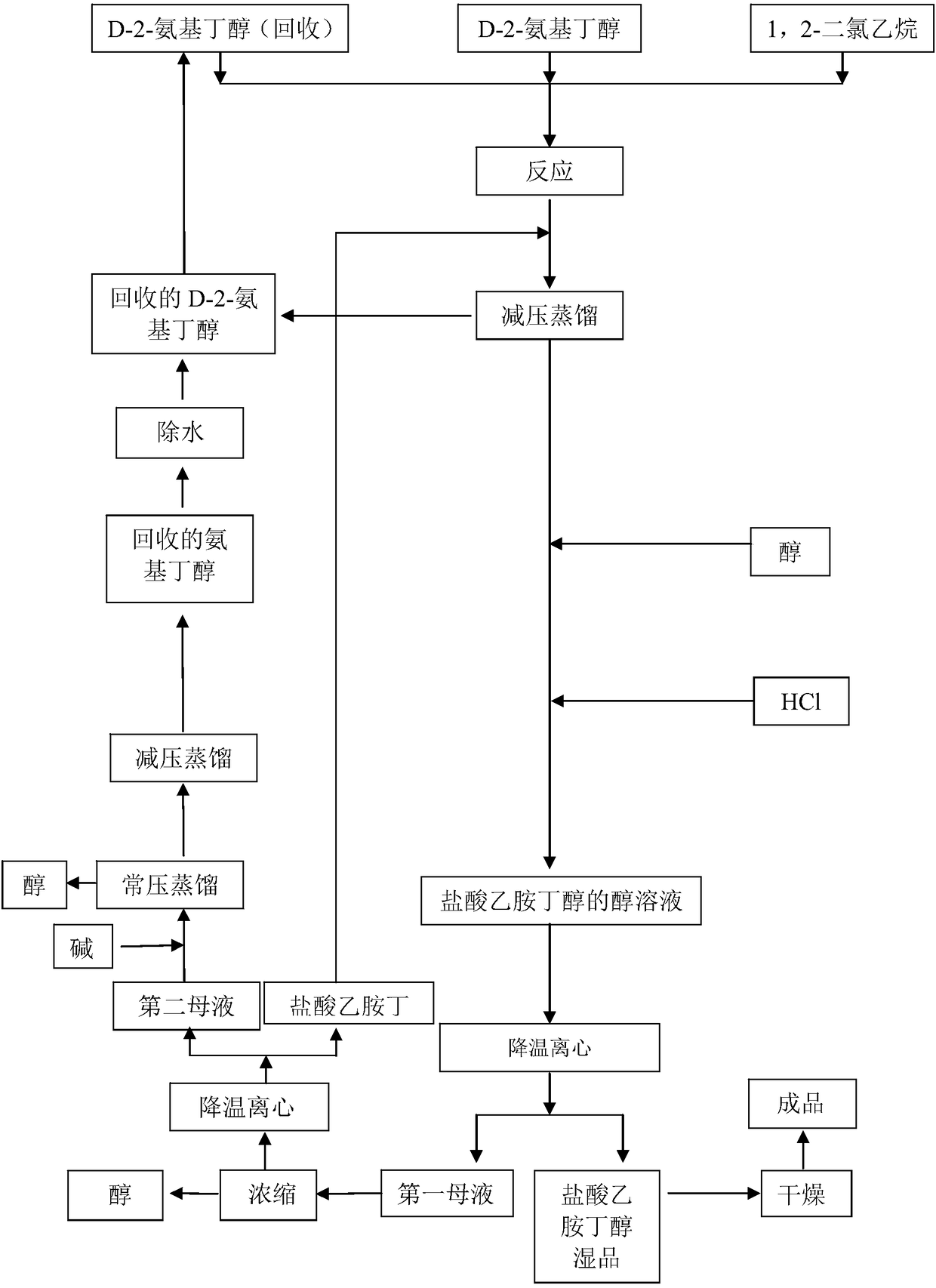
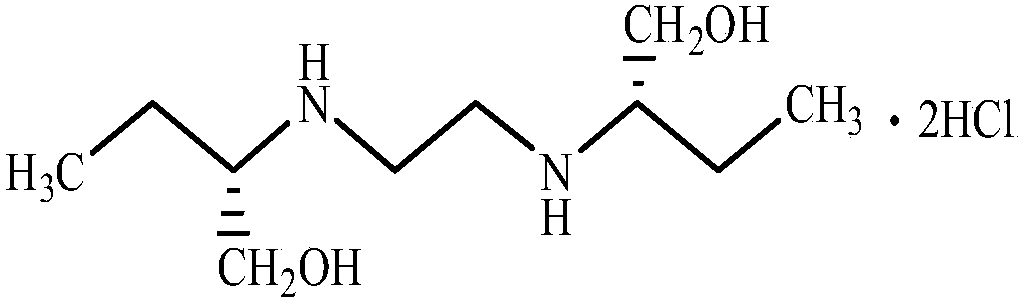
[0058] Put 295g (3.3094mol) of S-(+)-2-amino-1-butanol (specific rotation +10.1°) into a 500mL three-neck flask, stir and heat up to 110°C, and slowly add 35g ( 0.3536mol) of 1,2-dichloroethane (the feeding ratio of aminobutanol to 1,2-dichloroethane is 9.36:1), the temperature is controlled at 110°C-140°C within 2 hours, and then in In this temperature range, heat preservation reaction was carried out for 3 hours. At 150°C, 221.5 g (2.4849 mol) of S-(+)-2-amino-1-butanol was recovered by vacuum distillation, and the vacuum pressure was controlled at -0.09 MPa. Cool down to 70°C, add 200g of absolute ethanol, stir, slowly cool down to about 30°C, add dropwise 37.3g of hydrochloric acid ethanol (HCl content 30%), stir, and control the pH between 3 and 3.5. Slowly lower the temperature to 8°C to 10°C, and separate by suction filtration to obtain ethambutol hydrochloride 79.3g (yield 80.98%, m.p.199°C to 204°C), purity 99.9%, residue on ignition 0.01%, particle size 90 to 110 me...
Embodiment 2
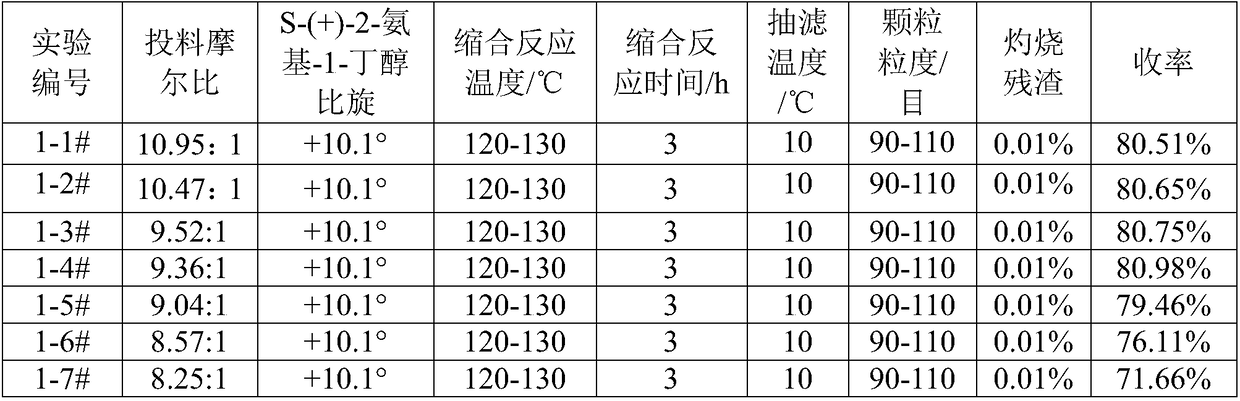
[0064] With reference to the feed ratio and reaction conditions of Example 1, the specific rotation of S-(+)-2-amino-1-butanol is changed separately, and other conditions are unchanged, to determine the impact on product yield and quality, see Table 2 .
[0065] Table 2: Effect of S-(+)-2-amino-1-butanol specific rotation on product yield and quality
[0066]
Embodiment 3
[0068] With reference to the reaction conditions of Example 1, the condensation reaction temperature and the condensation reaction time were changed, and other conditions were kept constant, so as to determine the influence on the product yield, as shown in Table 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com