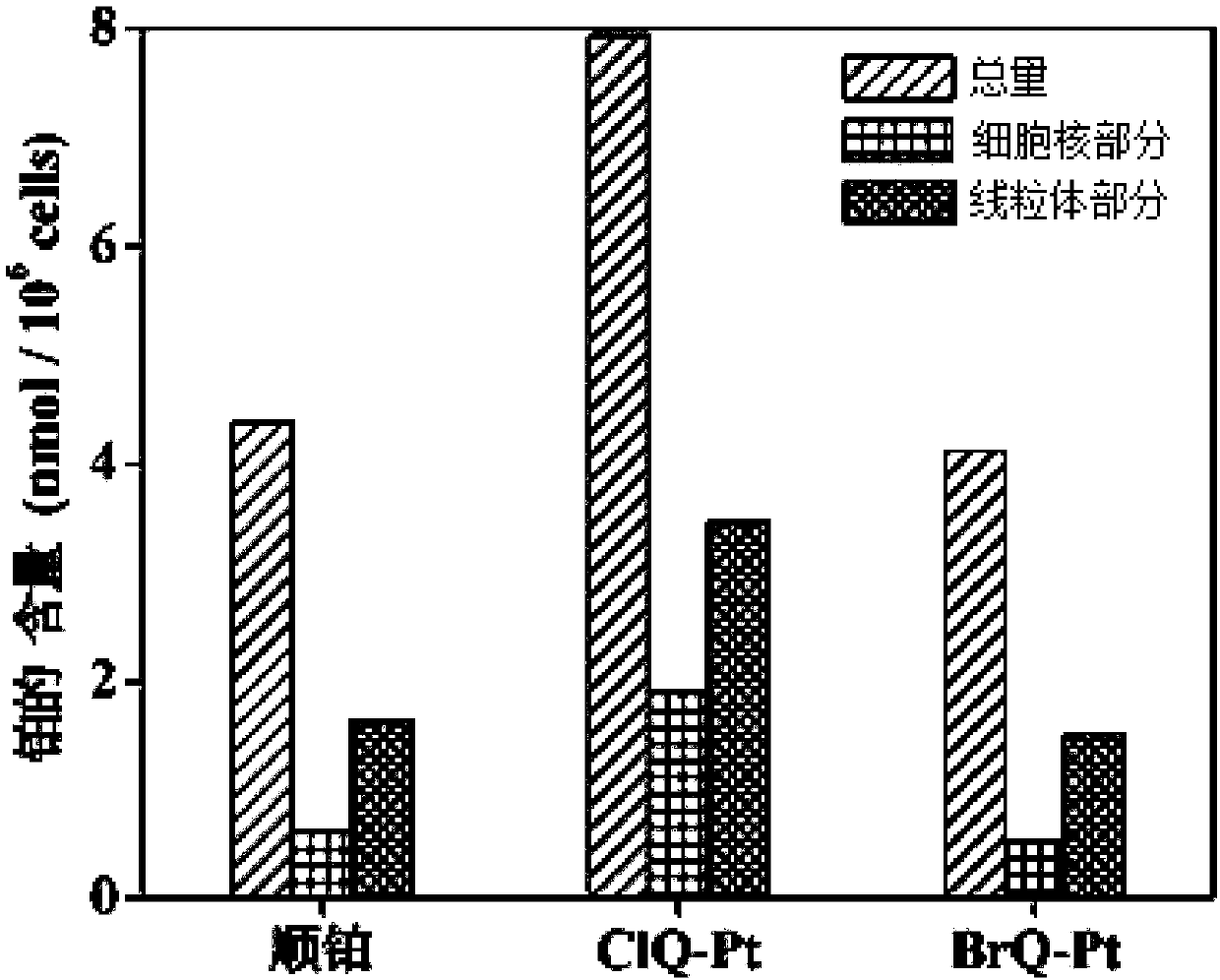
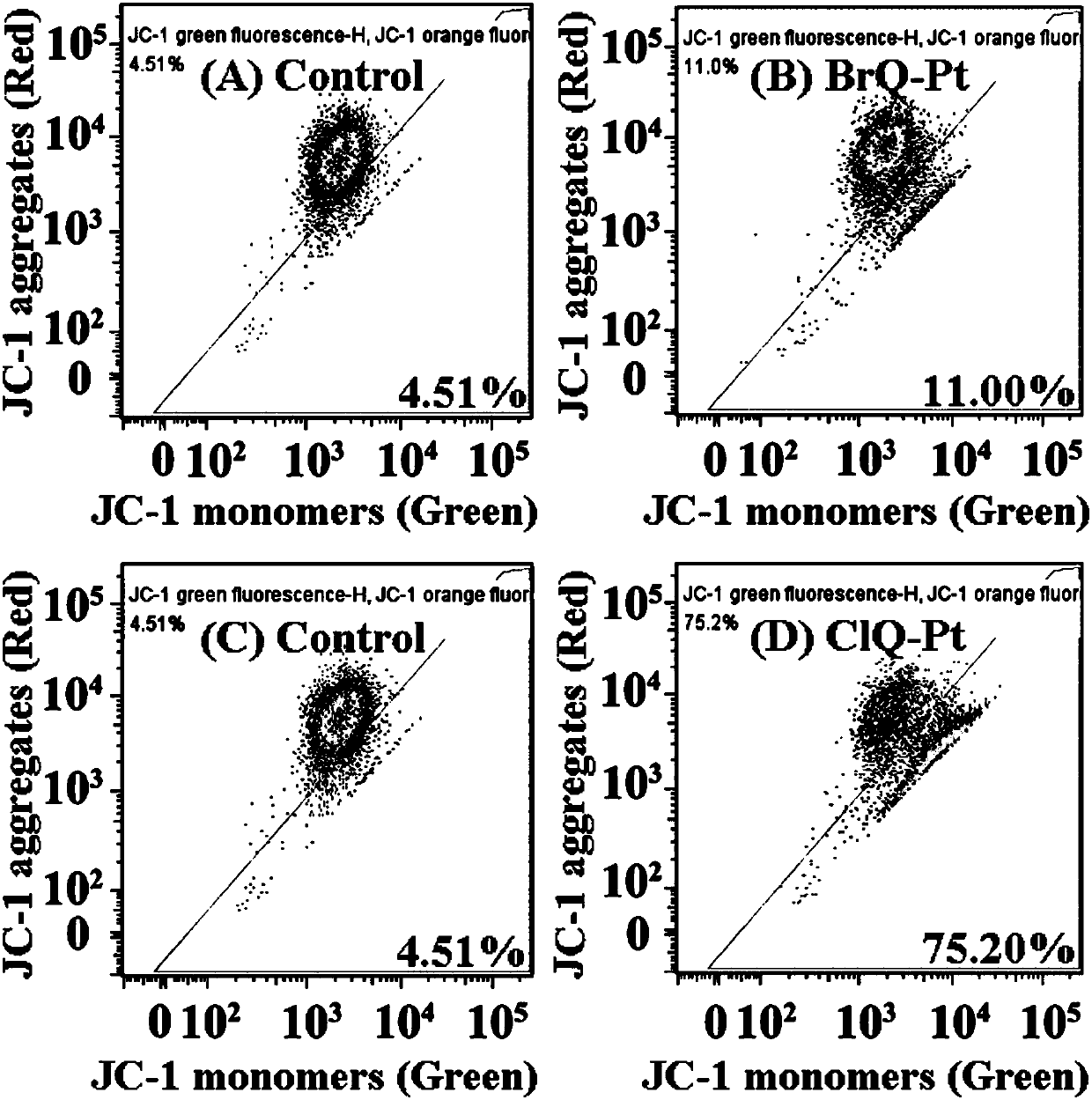
Halogenated 8-hydroxyquinoline platinum (II) complexes and synthesis method and application thereof
A synthetic method and compound technology, applied in the field of medicine, can solve the problem of high toxicity and achieve the effects of low toxicity, good proliferation inhibitory activity, and good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh H-ClQ and dichlorobis(dimethylsulfoxide)platinum(II) in the same amount, each 1mmol, dissolve H-ClQ in 50mL of 100v / v% methanol, and add dichloro· Di(dimethyl sulfoxide) platinum(II) was dissolved in 1.0 mL of DMSO, after the two solutions were mixed, reacted at 80°C for 12 hours, concentrated and evaporated to remove most of the solvent (85% of the solvent added) After cooling to room temperature and standing still, yellow crystals were precipitated, and the crystals were collected and dried to obtain a yellow solid product (yield 95.0%).
[0028] The products obtained in this implementation were characterized by infrared spectroscopy, nuclear magnetic resonance hydrogen / carbon spectroscopy, elemental analysis, electrospray mass spectrometry, and X-single crystal diffraction, as follows:
[0029] (1) Infrared spectrum:
[0030] IR(KBr): 3933,3846,3782,3696,3456,3011,2921,1584,1555,1500,1448,1370,1317,1268,1198,1138,1138,1026,971,947,922,865,828,771,691,537,517,438cm -1...
Embodiment 2
[0050] Weigh the same amount of H-BrQ and dichlorobis(dimethylsulfoxide) platinum(II), each 1mmol, and dissolve H-BrQ in 25mL of 75v / v% ethanol. Di(dimethylsulfoxide)platinum(II) was dissolved in 10.0mL of water. After the two solutions were mixed, they were reacted at 65°C for 24 hours, concentrated and evaporated to remove most of the solvent (70% of the solvent added). After cooling to room temperature and standing still, yellow crystals were precipitated, and the crystals were collected and dried to obtain a yellow solid product (yield 90.0%).
[0051] The products obtained in this implementation were characterized by infrared spectroscopy, nuclear magnetic resonance hydrogen / carbon spectroscopy, and electrospray mass spectrometry, as follows:
[0052] (1) Infrared spectrum:
[0053] IR (KBr): 3913,3782,3697,3463,3006,2919,1552,1494,1443,1369,1315,1272,1195,1147,1026,948,869,825,768,680,595,530,510,438cm-1.
[0054] (2) Proton nuclear magnetic resonance spectrum:
[0055] 1H NMR(5...
Embodiment 3
[0064] Weigh H-ClQ and dichloro-bis(dimethylsulfoxide)platinum(II) in the same amount, 1mmol each, and dissolve H-ClQ in 50mL of 90v / v% methanol. Di(dimethyl sulfoxide) platinum(II) was dissolved in 10 mL of 75v / v% ethanol, the two solutions were mixed, the resulting mixed solution was reacted at 45°C for 8 hours, concentrated and evaporated to remove 81% of the solvent, and then cooled After standing at room temperature, yellow crystals were precipitated, and the crystals were collected and dried to obtain a yellow solid product with a yield of 85.3%.
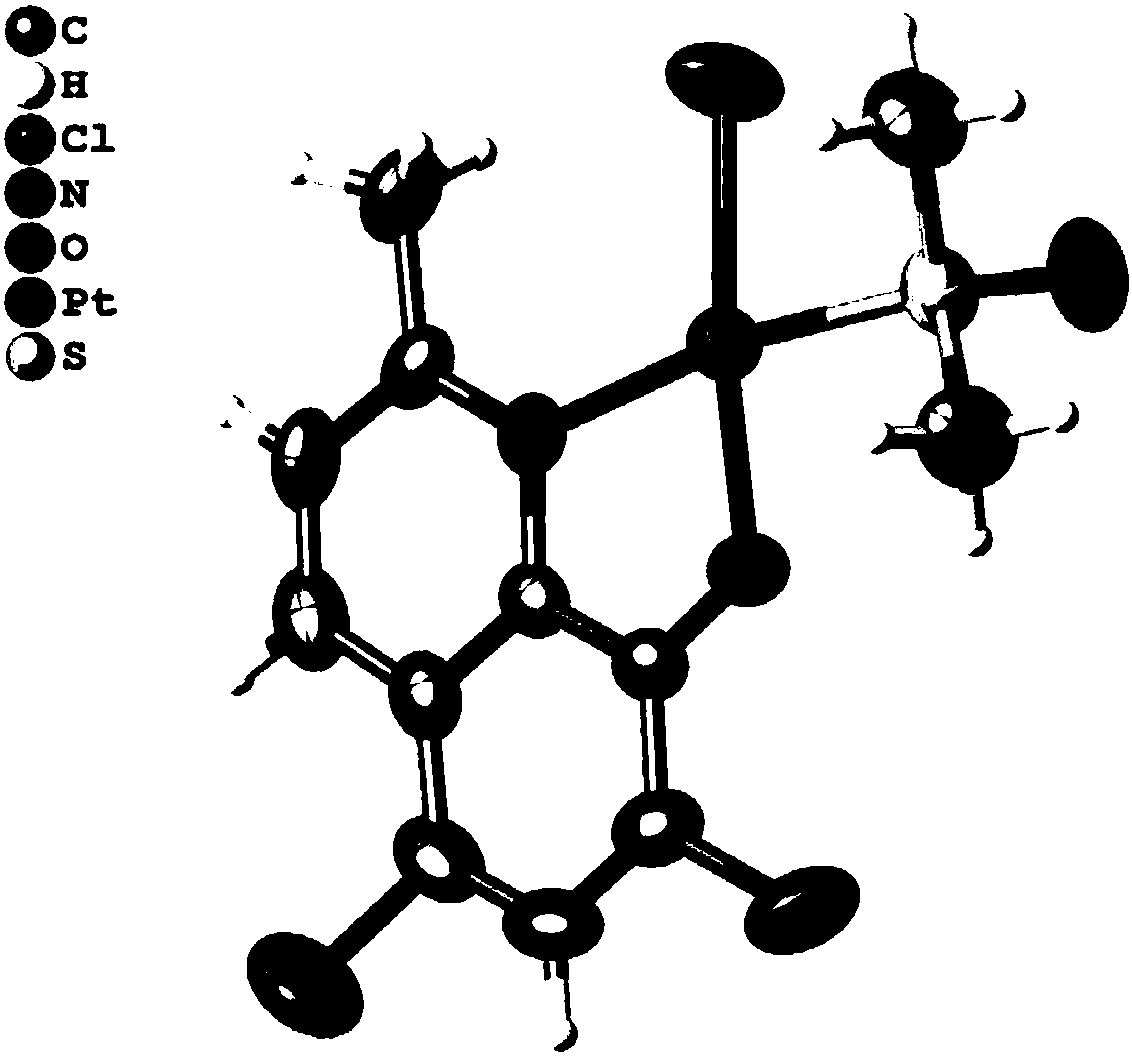
[0065] The product obtained in this implementation was characterized by infrared spectroscopy, nuclear magnetic resonance hydrogen / carbon spectroscopy, elemental analysis, X-single crystal diffraction and electrospray mass spectrometry, and it was determined to be the target product ClQ-Pt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com