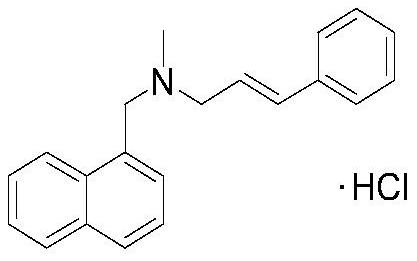
A kind of preparation method of naftifine hydrochloride
A technology of naftifine hydrochloride and acid-binding agent, which is applied in the field of preparation of naftifine hydrochloride, can solve the problems of high cost, low yield and low purity of naftifine hydrochloride, achieve simple steps, shorten process production time, and save raw materials cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
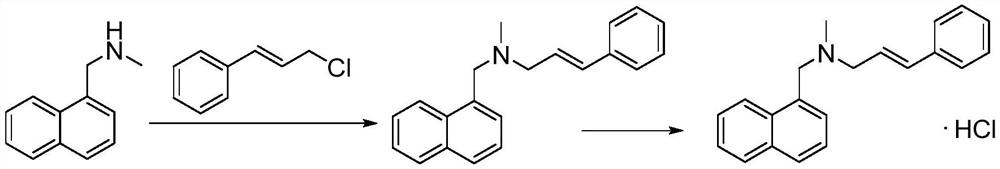
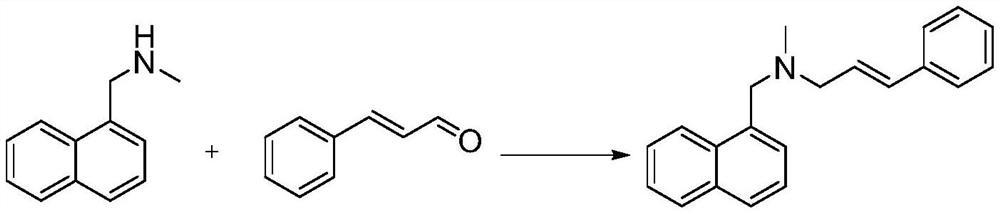
[0033] A preparation method of naftifine hydrochloride, comprising the following steps,
[0034] Step 1: Mix cinnamyl alcohol and dichloromethane to dissolve, control the temperature of the solution at 20-40°C, then add a chlorinated reagent and keep it warm for 2-6 hours, then add water to quench the reaction, then separate the organic phase, and use Wash the organic phase with saturated brine, let it stand, and then separate the organic phase and concentrate to obtain cinnamyl chloride;
[0035] Step 2: Mix the cinnamyl chloride in step 1 with the organic solvent as the reaction solvent, add dropwise to the methylamine solution, and keep it warm at 20-50°C for 1-5 hours, then concentrate to obtain a concentrate, and then add to the concentrate Dichloromethane was added to mix, filtered, and the filtrate was concentrated to obtain the crude product of trans-N-cinnamylmethylamine;
[0036] Step 3: Add toluene and acid-binding agent to the crude product of trans-N-cinnamylmeth...
Embodiment 1
[0057] A preparation method of naftifine hydrochloride, comprising the following steps:
[0058] Step 1: Dissolve cinnamyl alcohol (10g, 74.5mmol) in dichloromethane (50mL, 5V / w), stir evenly, control the temperature of the solution to 25°C, add thionyl chloride (13.3g, 111.8mmol) dropwise , dropwise, after 3 h of incubation at 25 ° C, add water (25 mL) to quench the reaction, separate the organic phase, and wash the organic phase with saturated brine (25 mL), let it stand, then separate the organic phase, and Concentrate under reduced pressure to obtain 11.2 g of brown liquid (cinnamyl chloride);
[0059] Step 2: Dissolve the brown liquid (cinnamyl chloride) in step 1 with absolute ethanol (11.2g) to obtain a feed solution. At 25°C, add the above feed solution dropwise to 45.6g of 30% methylamine ethanol solution ( Methylamine equivalent is about 6.0), dropwise, react for 2h, the reaction solution is concentrated under reduced pressure at 55°C until no liquid flows out, then...
Embodiment 2
[0066] A preparation method of naftifine hydrochloride, comprising the following steps:
[0067] Step 1: Dissolve cinnamyl alcohol (10g, 74.5mmol) in dichloromethane (50mL, 5V / W), stir evenly, control the temperature of the solution to 25°C, add thionyl chloride (16.0g, 89.1mmol) dropwise , dropwise, after 2h of heat preservation reaction at 25°C, add water (25mL) to quench the reaction, separate the organic phase, and then wash the organic phase with saturated brine (25mL), let it stand, separate the organic phase, and reduce Concentrate under reduced pressure to obtain 10.9 g of brown liquid (cinnamyl chloride).
[0068] Step 2: Dissolve the brown liquid (cinnamyl chloride) in step 1 with absolute ethanol (10.9g) to obtain a feed solution. At 25°C, add the above feed solution dropwise to 67.2g of 30% methylamine tetrahydrofuran solution ( Methylamine equivalent is about 8.0), dropwise, react for 2.5h, the reaction solution is concentrated under reduced pressure at 55°C unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com