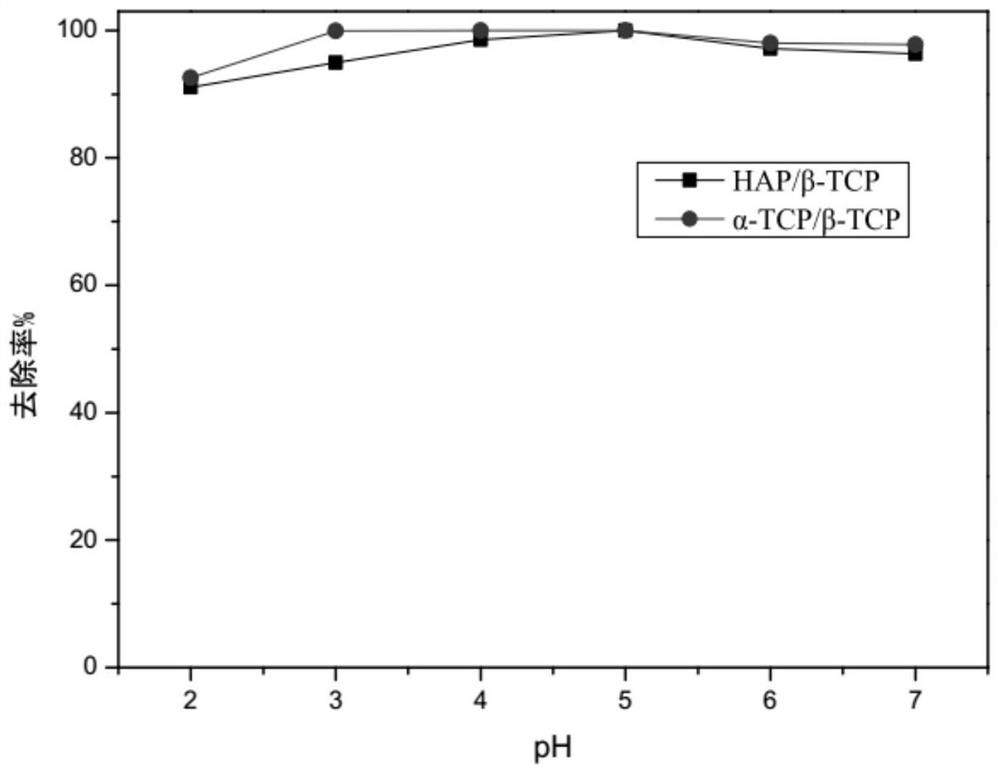
An application of using biphasic calcium phosphate to remove heavy metal lead in water
A biphasic calcium phosphate and heavy metal technology, applied in water/sewage treatment, water/sludge/sewage treatment, chemical instruments and methods, etc., can solve the problems of low removal efficiency and large amount of adsorbent, and achieve removal effect Good, high efficiency and stability, low environmental risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of biphasic calcium phosphate HA / β-TCP:
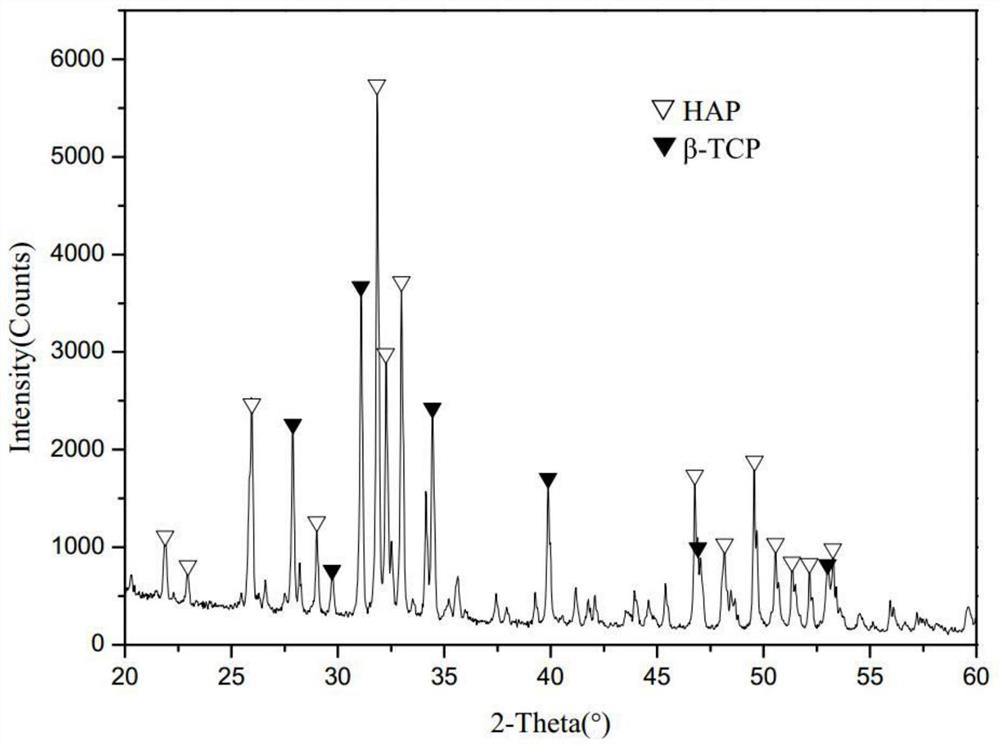
[0035] Ca(NO 3 ) 2 . 4H 2 O solution and (NH 4 ) 2 HPO 4 The solution was mixed according to the calcium-phosphorus molar ratio of 1.6, and the pH of the mixed solution was maintained at 11.0 to form a precursor, which was dried at 80°C and calcined at 900°C for 4 hours to obtain biphasic calcium phosphate powder HA / β-TCP. The X-ray diffraction pattern of biphasic calcium phosphate HA / β-TCP is as follows figure 1 as shown, figure 1 It shows that the diffraction peaks of the generated materials can basically overlap with the corresponding diffraction peaks of HA and β-TCP, indicating that biphasic calcium phosphate HA / β-TCP has been successfully prepared, and the particle size of biphasic calcium phosphate is 1-3 μm.
Embodiment 2
[0037] Preparation of biphasic calcium phosphate α-TCP / β-TCP:
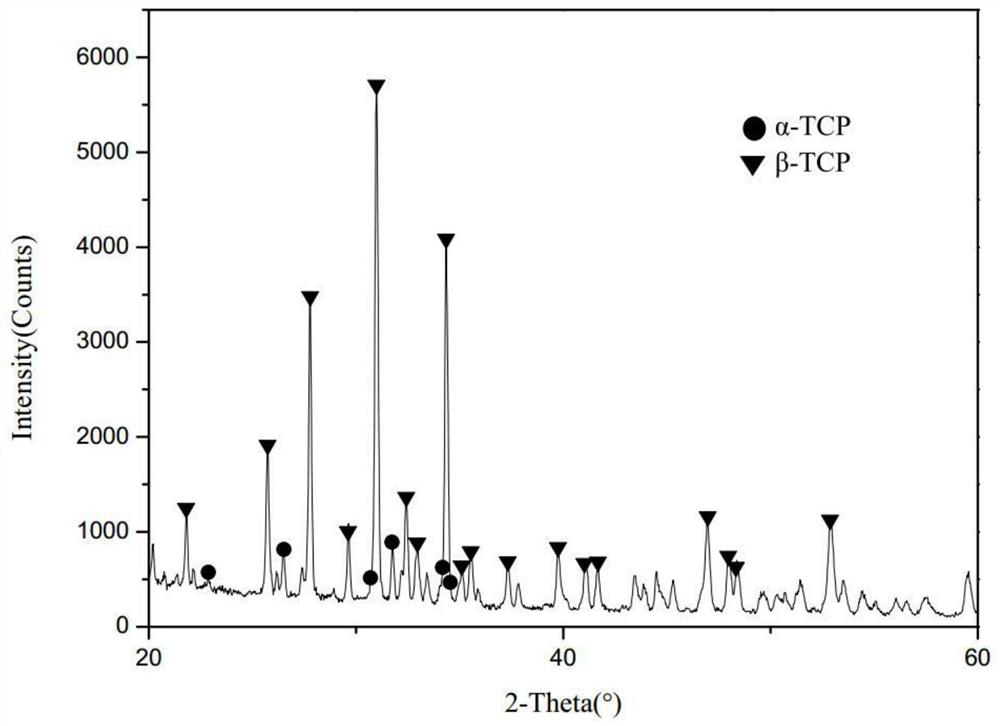
[0038] Ca(NO 3 ) 2 .4H 2 O solution and (NH 4 ) 2 HPO 4 The solution was mixed according to the calcium-phosphorus molar ratio of 1.5, and the pH of the mixed solution was maintained at 10.0 to form a precursor, which was dried at 80°C and calcined at 1120°C for 4 hours to obtain biphasic calcium phosphate powder α-TCP / β-TCP. The X-ray diffraction pattern of biphasic calcium phosphate α-TCP / β-TCP is as follows figure 2 as shown, figure 2 It shows that the diffraction peaks of the generated materials can basically overlap with the corresponding diffraction peaks of α-TCP and β-TCP, indicating that biphasic calcium phosphate α-TCP / β-TCP has been successfully prepared, and the particle size of biphasic calcium phosphate is 1-3 μm.
Embodiment 3
[0040] Preparation of biphasic calcium phosphate HA / β-TCP:
[0041] Ca(NO 3 ) 2 .4H 2 O solution and (NH 4 ) 2 HPO 4 The solution was mixed according to the calcium-phosphorus molar ratio of 1.5, and the pH of the mixed solution was maintained at 11.0 to generate a precursor, which was dried at 80°C and calcined at 1100°C for 3 hours to obtain biphasic calcium phosphate powder HA / β-TCP.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com