High-stability copper-based catalyst for preparing ethylene glycol by hydrogenating dimethyl oxalate and preparation method of high-stability copper-based catalyst
A technology of copper-based catalyst and dimethyl oxalate, which is applied in the preparation of hydroxyl compounds, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of stability degradation and improve stability , Improving the ability to resist hydrophilic substances and delaying the loss rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
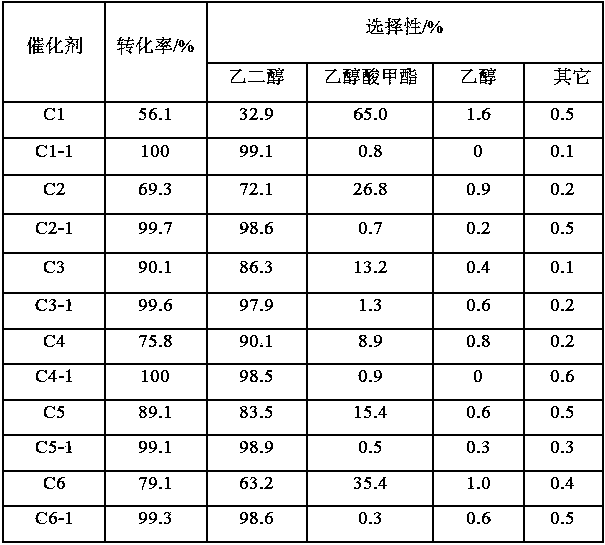
[0022] The preparation method of the highly stable copper-based catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol of the present embodiment is as follows:
[0023] (1) 21.1g Cu(NO 3 ) 2 ·3H 2 O and 5.2gNi(NO 3 ) 2 ·6H 2 After fully dissolving in 100.0g deionized water, add 60.0g ammonia water to make cuproammonia solution; add 80.0g25% silica sol and 50.0g20% zirconium sol into the solution, fully stir in a water bath at 50°C, and heat up to 90°C Distill ammonia at ℃, stop heating when the pH of the system is 7~8, and wash the obtained solid precipitate, and stop when the pH of the filtrate is 7. After drying the washed catalyst at 120°C for 24h, calcining at 450°C for 4h, and fully grinding to obtain unmodified copper-based catalyst C1;
[0024] (2) Weigh 5.0g of the above sample C1, put it into a round bottom flask, and add 40ml of toluene to it, slowly add 2.5ml of dimethyldiethoxysilane dropwise under stirring, continue to shake for a while, and h...
Embodiment 2
[0027] The preparation method of the highly stable copper-based catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol of the present embodiment is as follows:
[0028] (1) 16.4g Cu(NO 3 ) 2 ·3H 2 O and 1.66gCe (NO 3 ) 2 ·6H 2 O was dissolved in 50.0g of distilled water, fully dissolved and then added to the mixed solution formed by 42.0g of ethanol and 46.5g of ethyl orthosilicate, mechanically stirred the above mixed solution and placed in a 60°C water bath for 1.5h to form a sol-gel. After aging the gel at room temperature for 12 hours, cut it into thin slices, add an aqueous solution of dimethylamine with pH = 12, place the mixture in a hydrothermal kettle and seal it, then put it in an oven for 20 hours at 100 °C, and take out the reactant after the reaction is over The reaction kettle was treated in a water bath at 85°C for 20h, then moved into an oven and dried at 100°C for 24h, and the solid particles obtained after roasting and drying at 400°C in a...
Embodiment 3
[0032] The preparation method of the highly stable copper-based catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol of the present embodiment is as follows:
[0033] (1) 48.6gCu(NO 3 ) 2 ·3H 2 O and 3.6gH 3BO 3 After fully dissolving with 100.0g of deionized water, add 72.1g of urea to prepare a solution; add 48.0g of 25% alumina sol into the solution, stir fully in a water bath at 50°C, then raise the temperature to 90°C for uniform hydrolysis reaction, when the system Stop heating when the pH is 7-8, and wash the obtained solid precipitate, and stop when the pH of the filtrate is 7. After drying the washed catalyst at 110°C for 30h, calcining at 350°C for 10h, and fully grinding to obtain unmodified copper-based catalyst C3;
[0034] (2) Weigh 5.0g of the above sample C3, spread it evenly on a piece of filter paper, then put the filter paper on a self-made small shelf, and move them into the reaction kettle together, then add 3ml trimethyl chloride to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com