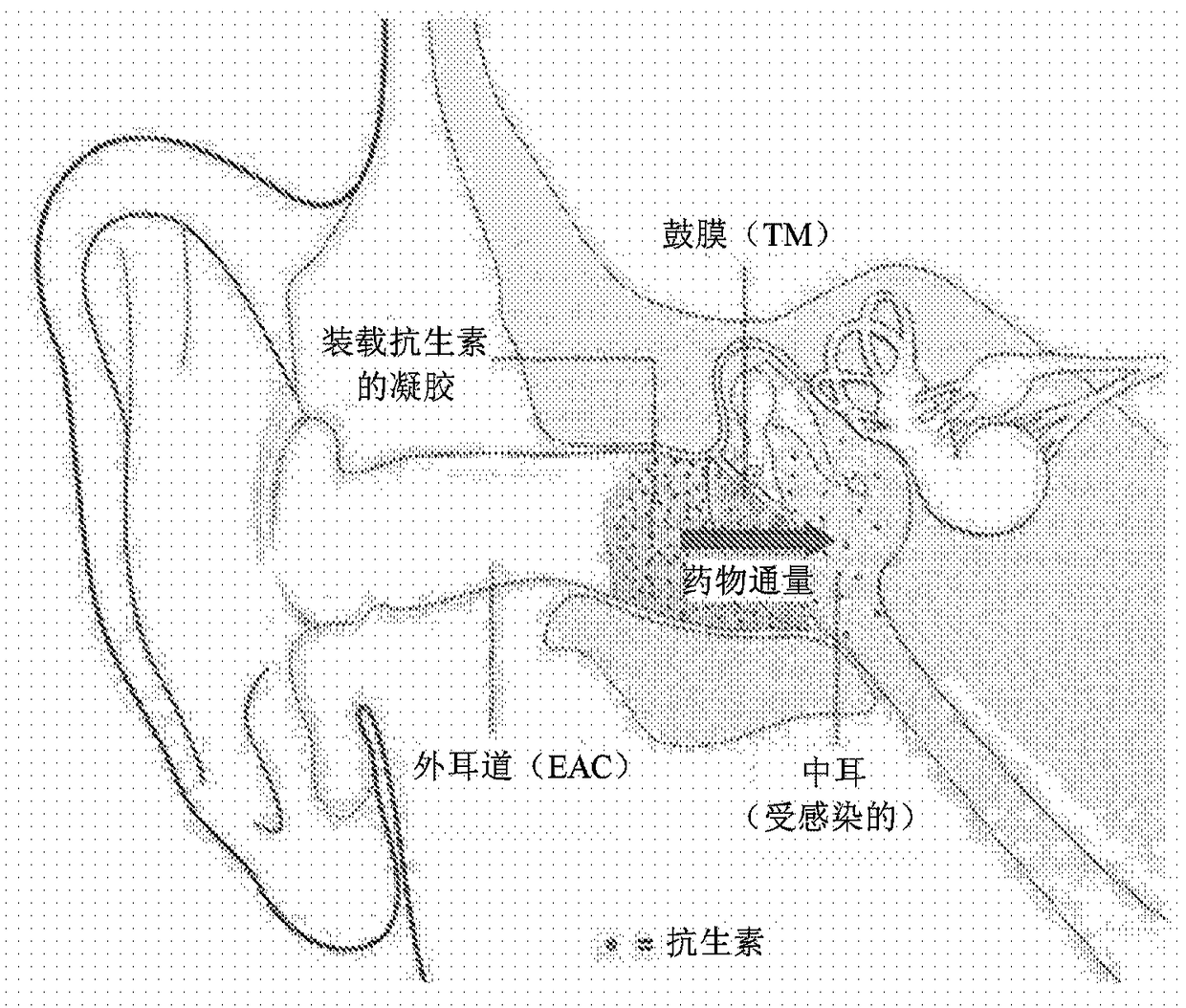
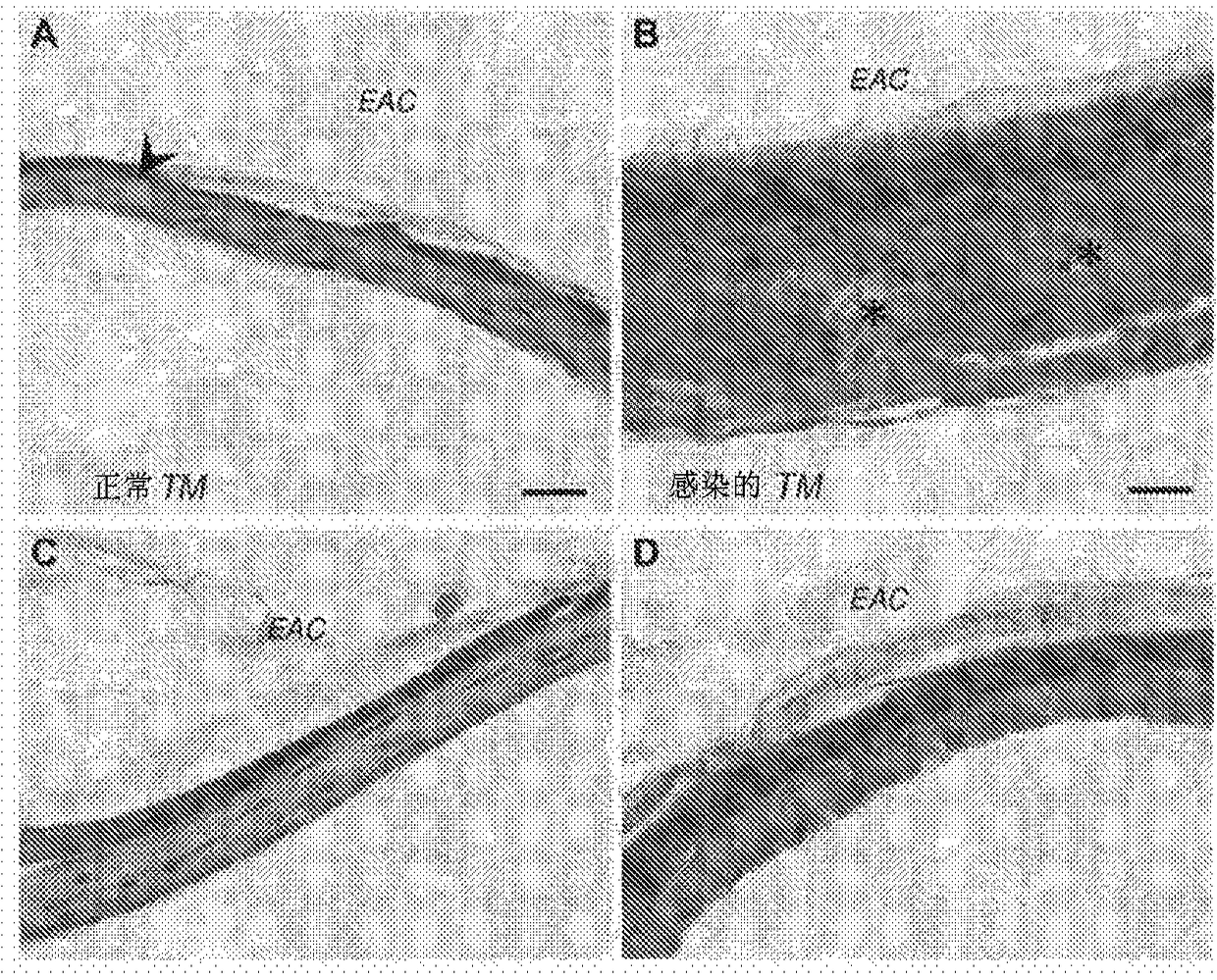
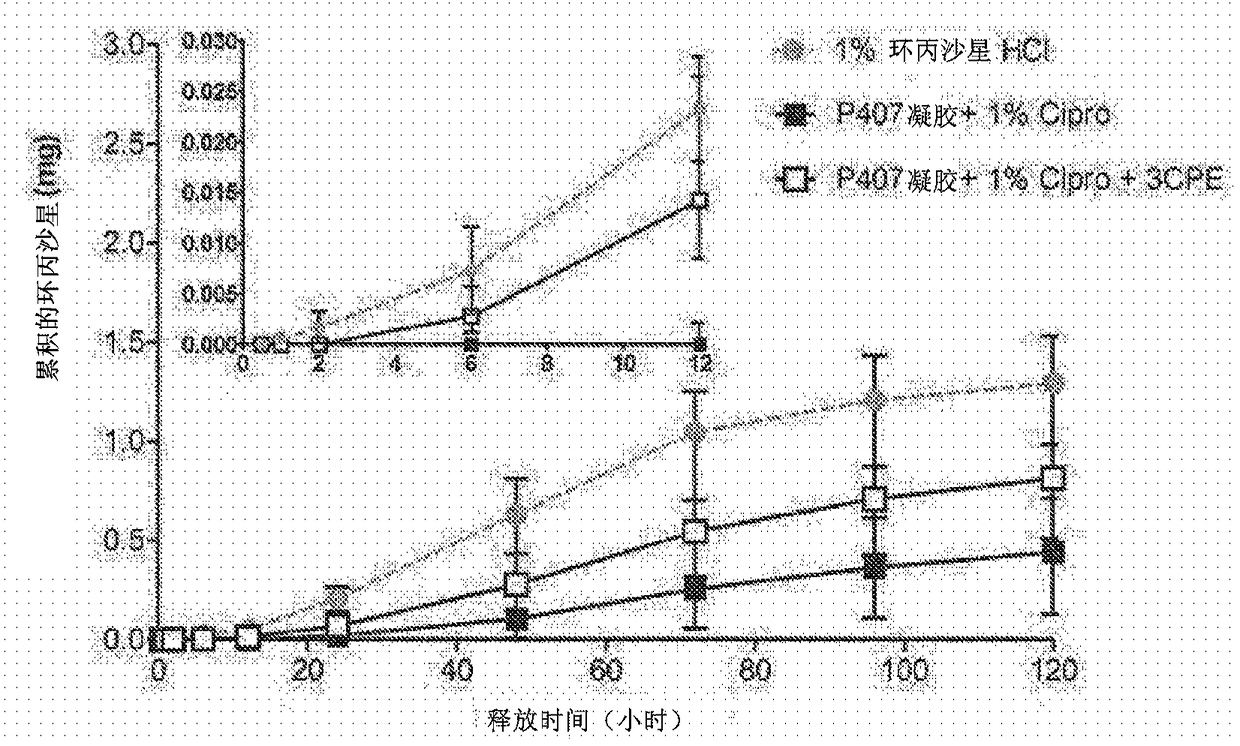
Compositions with permeation enhancers for drug delivery
A penetration enhancer and composition technology, applied in the direction of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0555] So that the invention described herein may be more fully understood, the following examples are set forth. The examples described in this application are provided to illustrate the compounds, pharmaceutical compositions and methods provided herein and are not to be construed in any way as limiting the scope thereof.
[0556] Materials and methods
[0557] Methods and design: The experiments compared the effects of the introduction of polymer matrix and CPE on TM permeability and OM healing rate. For ex vivo experiments, a sample size of 4 for each formulation was chosen, which provided 80% power to detect 50 based on power analysis using the nonparametric Friedman test (version 7.0, nQuery Advisor, Statistical Solutions, Saugus, MA). % flux difference. A sample size of 8-10 was used for in vivo experiments, which is supported by previous publications (Pelton et al., Antimicrob. Agents Chemother. 44, 654-657 (2000)). Comparisons between positive and negative potency r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com