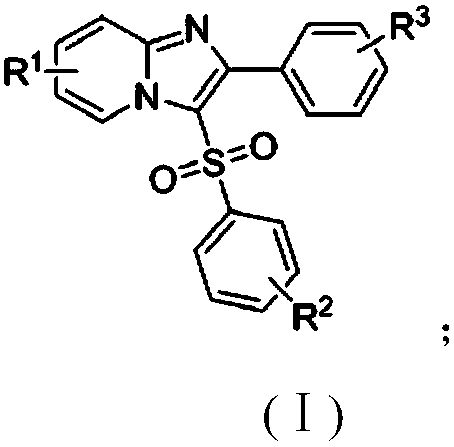
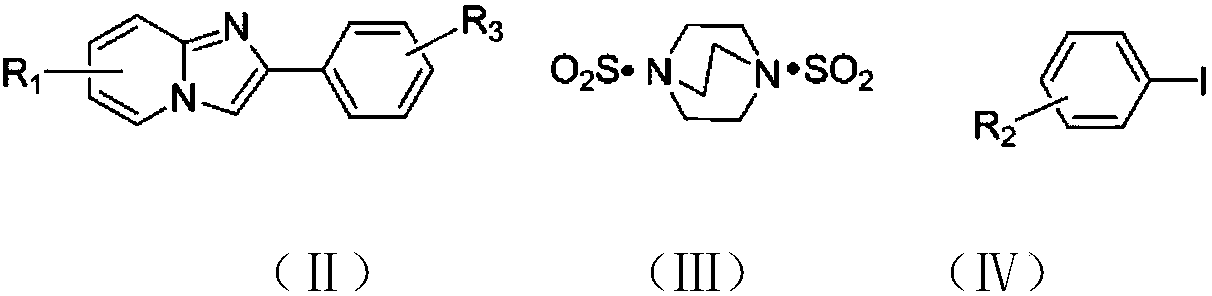
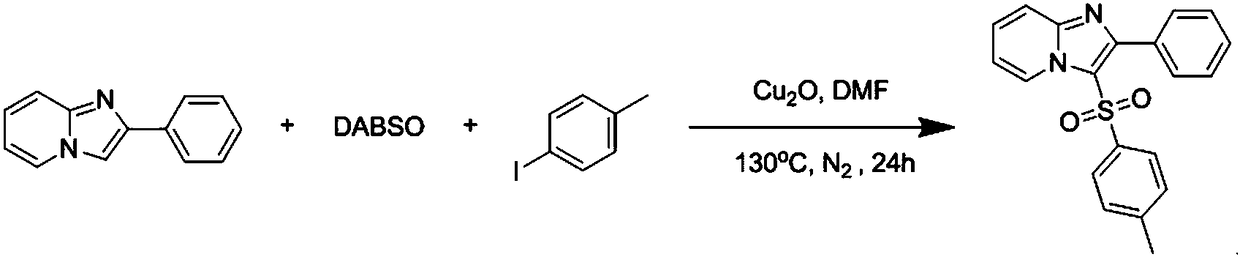
Copper catalytic synthesis method of organic sulphone compound and application
A synthesis method and technology for sulfone compounds, applied in the field of copper-catalyzed synthesis of organic sulfone compounds, can solve the problems of harsh reaction conditions, narrow substrate adaptability, cumbersome reaction steps, etc., and achieve low environmental pollution, easy operation, and functional group compatibility. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] At room temperature, charge cuprous oxide (0.02 mmol), 2-phenylimidazo[1,2-a]pyridine (0.2 mmol) into a 25 ml Schlenk tube equipped with a magnetic stirrer , p-methyliodobenzene (0.24 mmol), DABSO (1,4-diazabicyclo[2.2.2]octanebis(sulfur dioxide)) (0.3 mmol), 2 mL of anhydrous DMF was added under nitrogen, and the tube was placed Stir in an oil bath and react at 130°C for 24h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (6:1, v / v)) to obtain the target product 2-phenyl-3-toluenesulfonate Acyl imidazo[1,2-a]pyridine 49 mg, yield...
Embodiment 2
[0047]
[0048] At room temperature, charge cuprous oxide (0.02 mmol), 2-phenylimidazo[1,2-a]pyridine (0.2 mmol) into a 25 ml Schlenk tube equipped with a magnetic stirrer , p-chloroiodobenzene (0.24 mmol), DABSO (1,4-diazabicyclo[2.2.2]octane bis(sulfur dioxide)) (0.3 mmol), 2 mL of anhydrous DMF was added under nitrogen, and the tube was placed Stir in an oil bath and react at 130°C for 24h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specification: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (5:1, v / v)) to obtain the target product 3-(4-chlorophenylsulfone Acyl)-2-phenylimidazo[1,2-a]pyridine 56 mg,...
Embodiment 3
[0051]
[0052] At room temperature, charge cuprous oxide (0.02 mmol), 2-phenylimidazo[1,2-a]pyridine (0.2 mmol) into a 25 ml Schlenk tube equipped with a magnetic stirrer , p-bromoiodobenzene (0.24 mmol), DABSO (1,4-diazabicyclo[2.2.2]octanebis(sulfur dioxide)) (0.3 mmol), add 2 mL of anhydrous DMF under nitrogen, put the tube into Stir in an oil bath and react at 130°C for 24h. The progress of the reaction was monitored by TLC (Thin Layer Chromatography). After the reaction was completed, the resulting solution was cooled to room temperature, 5 ml of water and 20 ml of ethyl acetate were added to the reaction solution for extraction 4 times, and the organic phase was removed from the solvent by a rotary evaporator. The residue was purified with a silica gel column (silica gel specifications: 200 mesh to 300 mesh, eluent petroleum ether / ethyl acetate (6:1, v / v)) to obtain the target product 3-(4-bromophenylsulfone Acyl)-2-phenylimidazo[1,2-a]pyridine 61 mg, yield 74%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com