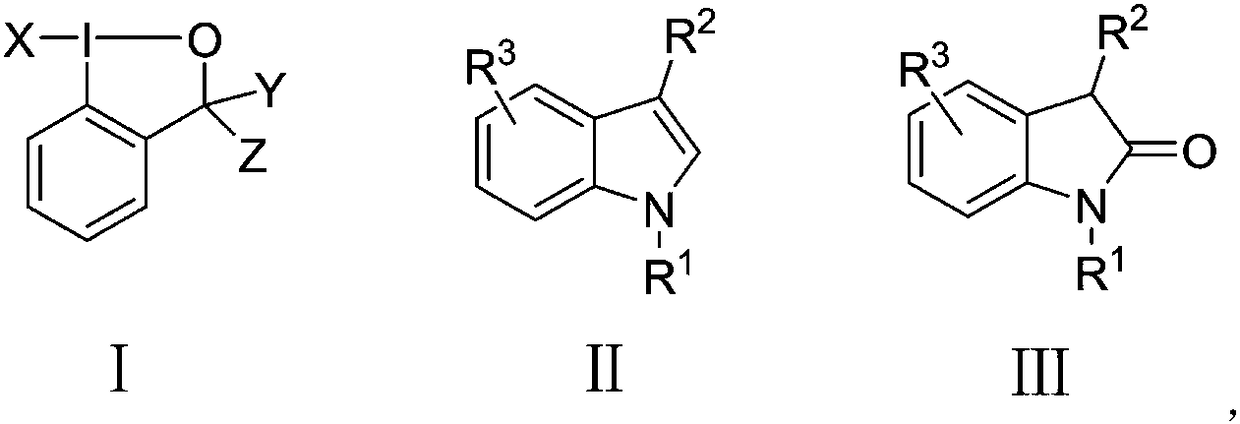
Method for directly synthesizing substituted 2-indolone by substituted indole under neutral condition
A neutral-condition, indolinone technology is applied in the field of directly synthesizing substituted 2-indolinone under neutral conditions for substituted indole, and achieves the effects of high yield, simple operation and high-efficiency preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Synthesis of 1-methyl-1,3-dihydroindolin-2-one with 1-methylindole as substrate
[0017] Add 0.4mL of 1,4-dioxane to a 50mL reaction tube at room temperature, then add 2mL of water, stir and mix well, weigh 1-fluoro-1,2-phenyliodohydrin-3-(1H)- Ketone (175mg, 0.66mmol) was added to the reaction tube and stirred for 1min, then the reaction tube was placed in an oil bath at 140°C, N-methylindole (78mg, 0.6mmol) was added, and a built-in condenser was added, and the reaction tube was heated at 140°C The reaction was carried out in an oil bath at ℃ for 5 hours, and the TLC spot plate showed that the reaction of the raw materials was complete. Take the reaction tube out of the oil bath, cool to room temperature, add 10 mL of saturated sodium bicarbonate to quench the reaction, then extract with 20 mL of ethyl acetate, combine the organic layers and dry them with anhydrous sodium sulfate. Analysis and separation gave 79 mg of yellow solid N-methyl-2 indolinone with...
Embodiment 2
[0018] Example 2 Synthesis of 1-methyl-1,3-dihydroindolin-2-one with 1-methylindole as substrate
[0019] Add 0.4mL of 1,4-dioxane to a 50mL reaction tube at room temperature, then add 2mL of water, stir and mix well, weigh 1-chloro-1,2-phenyliodohydrin-3-(1H)-one (187mg , 0.66mmol) was added to the reaction tube and stirred for 1min, then the reaction tube was placed in a 140°C oil bath, N-methylindole (78mg, 0.6mmol) was added, and a built-in condenser was added, and the reaction tube was placed in a 140°C oil bath Reacted in medium for 5 hours, TLC spot plate showed that the raw materials had reacted completely, took the reaction tube out of the oil bath, cooled to room temperature, added 10mL saturated sodium bicarbonate to quench the reaction, then extracted with 20mL ethyl acetate, combined the organic layers with anhydrous Na2SO4 dried. The organic layer was concentrated by rotary evaporation, and separated by column chromatography to obtain 55 mg of yellow solid N-met...
Embodiment 3
[0020] Example 3 Synthesis of 1-methyl-1,3-dihydroindolin-2-one with 1-methylindole as substrate
[0021] Add 2 mL of tetrahydrofuran to a 50 mL reaction tube at room temperature, then add 2 mL of water, and stir to mix. Weigh 1-chloro-1,2-phenyliodohydrin-3-(1H)-one (187mg, 0.66mmol) into the reaction tube and stir for 1min, then place the reaction tube in an oil bath at 40°C, add N- Methylindole (78mg, 0.6mmol) was reacted in an oil bath at 40°C for 8 hours with a built-in condenser. TLC spotting showed that the reaction of the raw materials was complete. Take the reaction tube out of the oil bath, cool to room temperature, and add 10mL The reaction was quenched with saturated sodium bicarbonate, extracted with 20 mL of ethyl acetate, and the combined organic layers were dried over anhydrous sodium sulfate. The organic layer was concentrated by rotary evaporation, and separated by column chromatography to obtain 24 mg of yellow solid N-methyl-2-indolinone with a yield of 27...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com