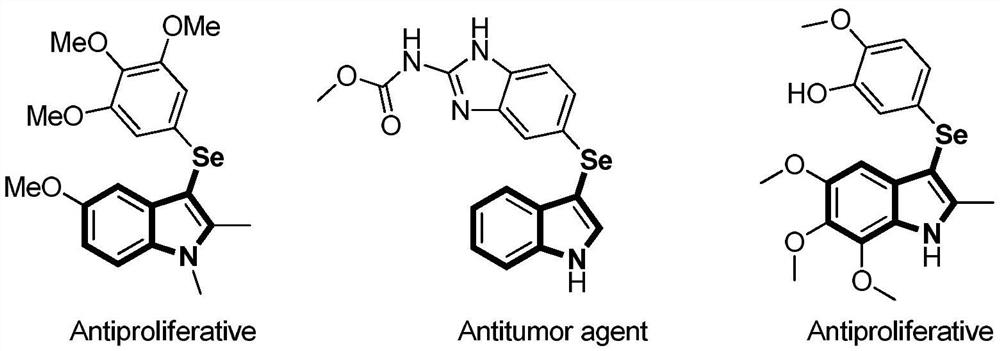
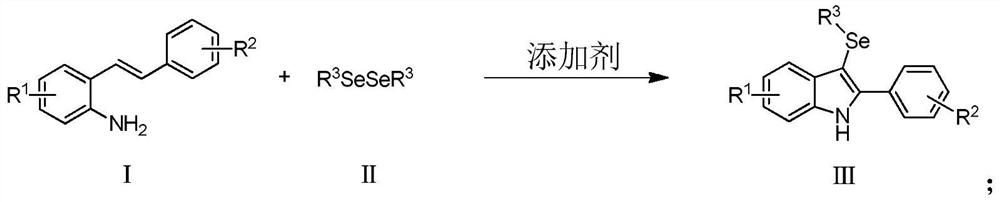
Method for synthesizing 3-selenoindole derivative
A technology of selenoindole derivatives and derivatives, applied in the field of synthesizing 3-selenoindole derivatives, capable of solving problems such as harsh conditions, complex processes, and limited sources of complex catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of 2-phenyl-3-(phenylselenyl)indole, structural formula is as follows:
[0046]
[0047] Under air atmosphere, raw materials 2-styrylaniline (0.2mmol), diphenyldiselenide (0.2mmol), additives NFSI (0.4mmol), pyridine (2mL) were added, reacted at 100°C for 5h, and the product was separated by 86%.
[0048] 1 H NMR (500MHz, CDCl 3 )δ8.50(s,1H),7.78-7.76(m,3H),7.50-7.43(m,4H),7.37-7.34(m,1H),7.31-7.27(m,3H),7.21-7.15( m,3H); 13 C NMR (125MHz, CDCl 3 )δ 142.0, 136.1, 134.0, 132.0, 131.9, 129.0, 128.5, 128.5, 128.3, 125.4, 123.2, 121.0, 120.8, 111.0, 95.8.
Embodiment 2
[0050] The preparation of 2-(4-tert-butylphenyl)-3-(phenylselenyl) indole, the structural formula is as follows:
[0051]
[0052] Under air atmosphere, add raw materials 2-(4-tert-butylstyryl)aniline (0.2mmol), diphenyldiselenide (0.2mmol) and additives NFSI (0.4mmol), pyridine (2mL), and react at 120°C After 6h, the product separation yield was 94%.
[0053] 1 H NMR (400MHz, CDCl 3 )δ8.53(s,1H),7.71-7.69(m,3H),7.50-7.45(m,3H),7.32-7.28(m,1H),7.25-7.19(m,3H),7.18-7.12( m,3H),1.38(s,9H); 13 C NMR (125MHz, CDCl 3 )δ151.7, 142.1, 136.1, 134.2, 132.2, 129.1, 129.0, 128.2, 128.1, 125.6, 125.3, 123.1, 121.0, 120.8, 110.9, 95.3, 34.7, 31.2.
Embodiment 3
[0055] The preparation of 2-naphthyl-3-(phenylselenyl)indole, structural formula is as follows:
[0056]
[0057] Under air atmosphere, add raw material 2-navinylaniline (0.2mmol), diphenyldiselenide (0.2mmol) and additives NFSI (0.4mmol), pyridine (2mL), react at 120°C for 5h, and the isolated yield of the product is 95%. %.
[0058] 1 H NMR (400MHz, CDCl 3 )δ8.59(s,1H),8.15(s,1H),7.88-7.82(m,4H),7.76(d,J=8.0Hz,1H),7.54-7.51(m,2H),7.46(d ,J=8.0Hz,1H),7.35-7.29(m,3H),7.24(d,J=7.2Hz,1H),7.19-7.13(m,3H); 13 C NMR (125MHz, CDCl 3 )δ 141.9, 136.3, 134.1, 133.1, 133.1, 132.2, 129.4, 129.0, 128.5, 128.3, 128.2, 127.8, 127.7, 126.6, 126.5, 126.0, 125.5, 123.3, 121.1, 120.9, 121.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com