Method for preparing ester by catalyzing halogenated aromatic and carbonyl source through non-transition metal under visible light
A non-transition metal, halogenated aromatic hydrocarbon technology, applied in the field of photocatalytic organic synthesis, to achieve the effect of mild reaction conditions, conducive to popularization and application, and wide applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
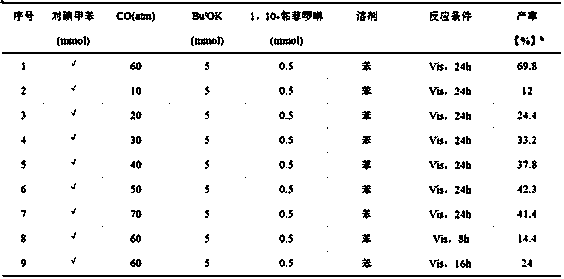
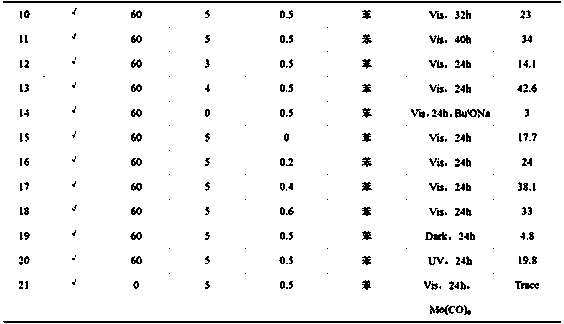
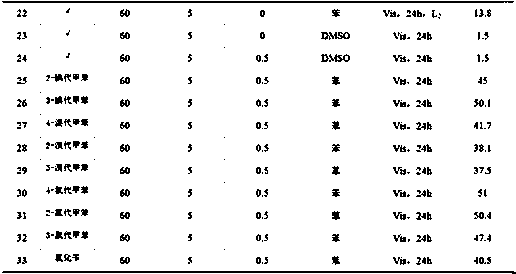
[0027] A non-transition metal visible light catalyzed method for preparing esters from halogenated aromatic hydrocarbons and carbonyl sources, the specific steps are:
[0028] 1) Add 561mg (5mmol) of potassium tert-butoxide, 90mg (0.5mmol) of 1,10-phenanthroline, 4ml of anhydrous benzene, and 1mmol of p-iodotoluene into a clean, dry, high-pressure reactor with magnets;
[0029] 2) After adding the sample, seal the reactor well, and move it from the glove box to the fume hood. Note that the follow-up operations are all carried out in the fume hood. First, flush the reactor with a certain pressure of CO gas to replace the nitrogen in the reactor. , after flushing three times, fill the reactor with 60atmCO gas, and close the two-way valve;
[0030] 3) Start stirring and carry out visible light reaction with xenon lamp (420nm2 ), after 24 h of reaction, stop the light and stirring, and release the CO gas in the reactor.
Embodiment 2
[0032] The specific experimental method is basically the same as in Example 1 of this part, except that the carbon monoxide pressure of 60 atm is changed to 10 atm.
Embodiment 3
[0034] The specific experimental method is basically the same as in Example 1 of this part, except that the carbon monoxide pressure of 60 atm is changed to 20 atm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com