Fully humanized single-domain antibody for human TIM-3 (T cell immunoglobulin and mucin-3) and applications
A technology of TIM-3 and single domain antibody, which is applied in the direction of application, antibody, antibody mimic/scaffold, etc. It can solve the problems of monoclonal antibody with large molecular weight, affecting therapeutic effect, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
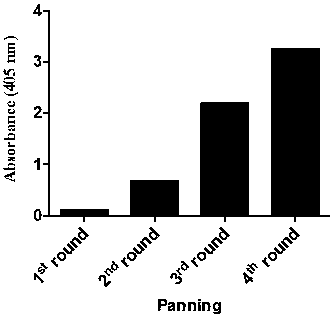
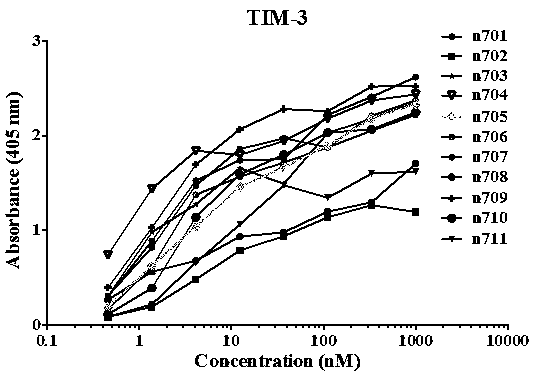
[0070] 1. Screening of TIM-3 specific single domain antibody (VH)
[0071] Using human VH 3-23 subfamily germline antibody as a template, using innovative design and antibody engineering technology to introduce heavy chain CDR regions (CDR1, CDR2, CDR3), constructing an actual measurement library with a capacity of 150 billion (1.5×10 11 ) super-large fully human single domain antibody library. Use this phage display single domain antibody library to screen antibodies against biotinylated TIM-3 protein. We immobilized biotin-labeled TIM-3 protein on streptavidin-coated magnetic beads, 10 12 Antibodies displayed by each phage were incubated with 5, 4, 2, and 1 micrograms of antigen for two hours at room temperature in rounds 1, 2, 3, and 4, respectively, and 10 phages were used for each round of screening. 12 indivual. Antibody enrichment was detected using a polyclonal phage ELISA. The first, second, third, and fourth rounds of phage were incubated with the coated protein...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com