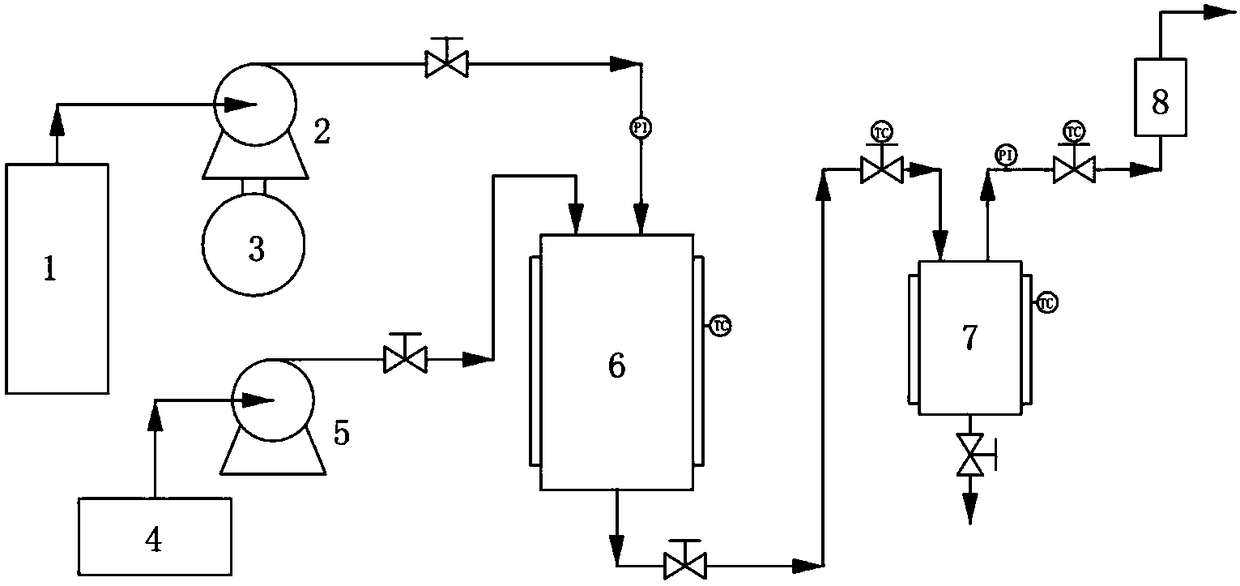
Method for preparing azilsartan solid dispersion by supercritical anti-solvent method
A supercritical anti-solvent, solid dispersion technology, applied in the field of medicine, can solve the problems of inappropriate preparation of azilsartan solid dispersion, and achieve the effects of outstanding substantive characteristics, improved dissolution performance, and significant progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
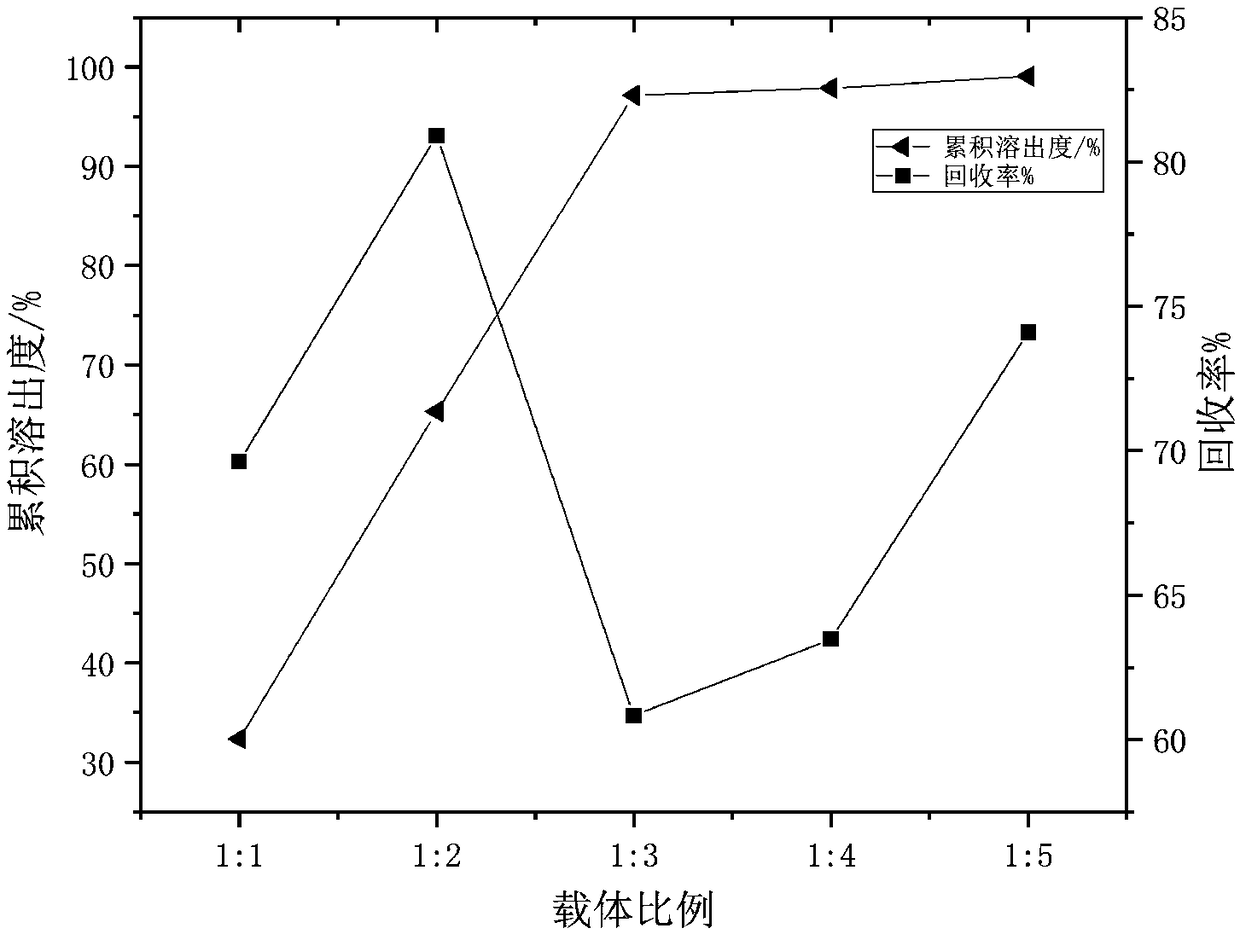
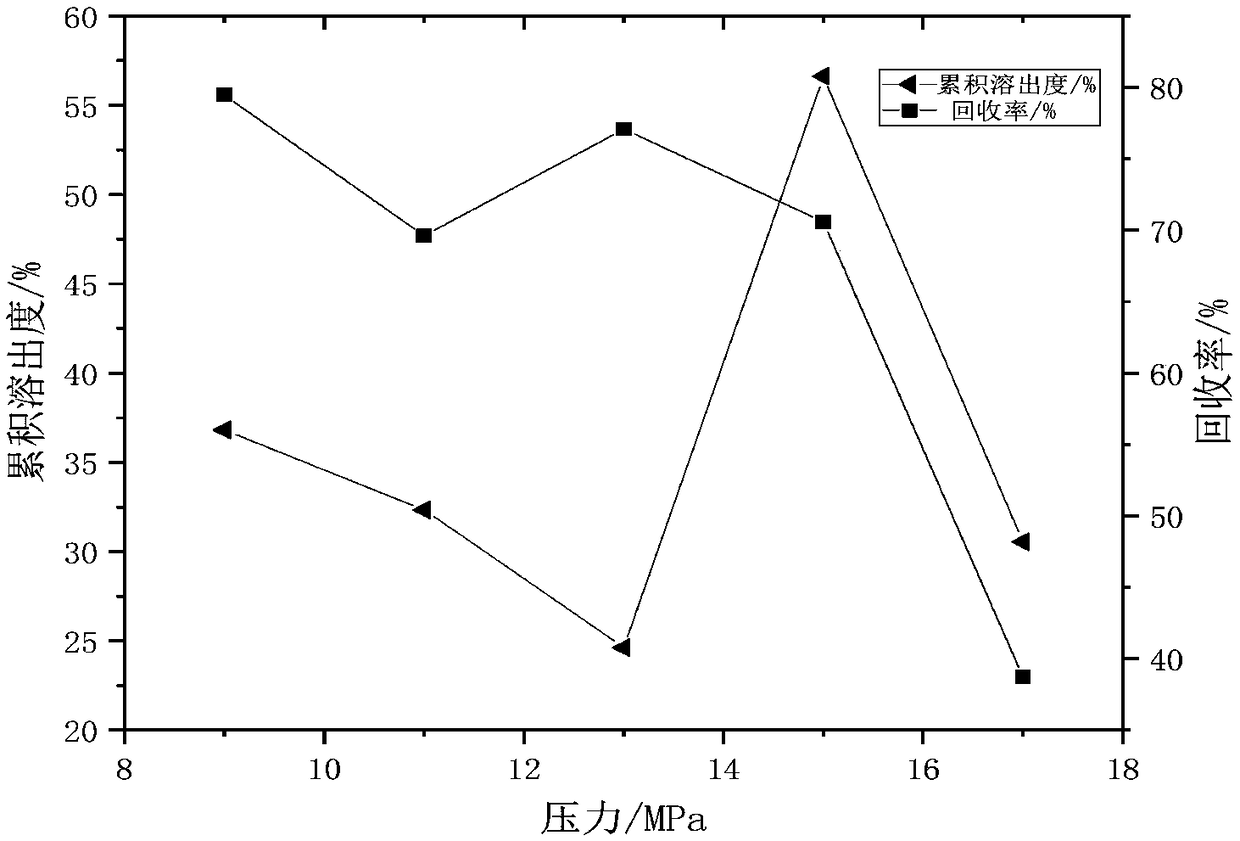
[0033] Embodiment 1: Single factor method investigates the impact of each parameter value range on cumulative dissolution rate and recovery rate
[0034] Experimental Instruments and Materials
[0035] The main instruments used in the experiment are shown in Table 1, and the main raw materials and reagents are shown in Table 2.
[0036] Table 1 Main Instruments
[0037] device name
model
Manufacturer
Supercritical Particle Preparation System
AppliedSeparations, USA
Series1500 high pressure infusion pump
AppliedSeparations, USA
air compressor pump
TYW-2
Suzhou Tongtong Electromechanical Co., Ltd.
Low temperature constant temperature bath
SDC-6
Nanjing Xinchen Biotechnology Co., Ltd.
UV-visible spectrophotometer
UV-1800
Shimadzu Corporation
Intelligent Dissolution Tester
ZRS-8L
Tianjin Tianda Tianfa Technology Co., Ltd.
Analytical Balances
BS124S
...
Embodiment 2
[0061] Embodiment 2: Application of supercritical antisolvent method to prepare preferred parameters of azilsartan solid dispersion
[0062] The method for preparing azilsartan solid dispersion by supercritical antisolvent method comprises the steps:
[0063] Step S1, dissolving azilsartan and a water-soluble carrier in an organic solvent to obtain azilsartan-carrier mixed solution;
[0064] Step S2, the CO 2 Pass into the crystallization kettle, adjust the temperature and pressure in the crystallization kettle;
[0065] Step S3, continue to feed CO 2 , maintaining the temperature and pressure in the crystallization tank constant, while passing the azilsartan-carrier mixed solution prepared in step S1 into the crystallization tank;
[0066] Step S4, after the azilsartan-carrier solution is passed through, continue to pass through CO 2 , keep the temperature and pressure in the crystallization kettle constant, and release the pressure after a period of time; when the pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com