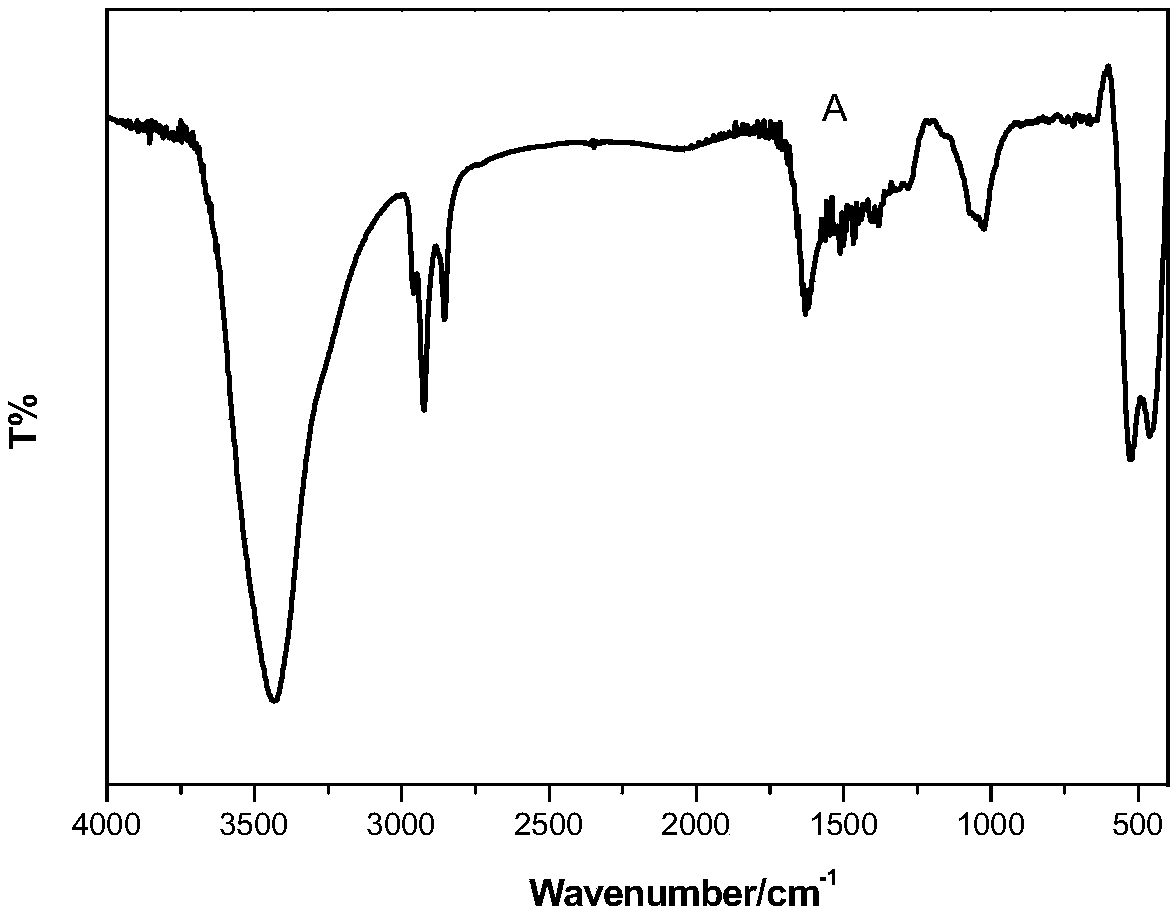
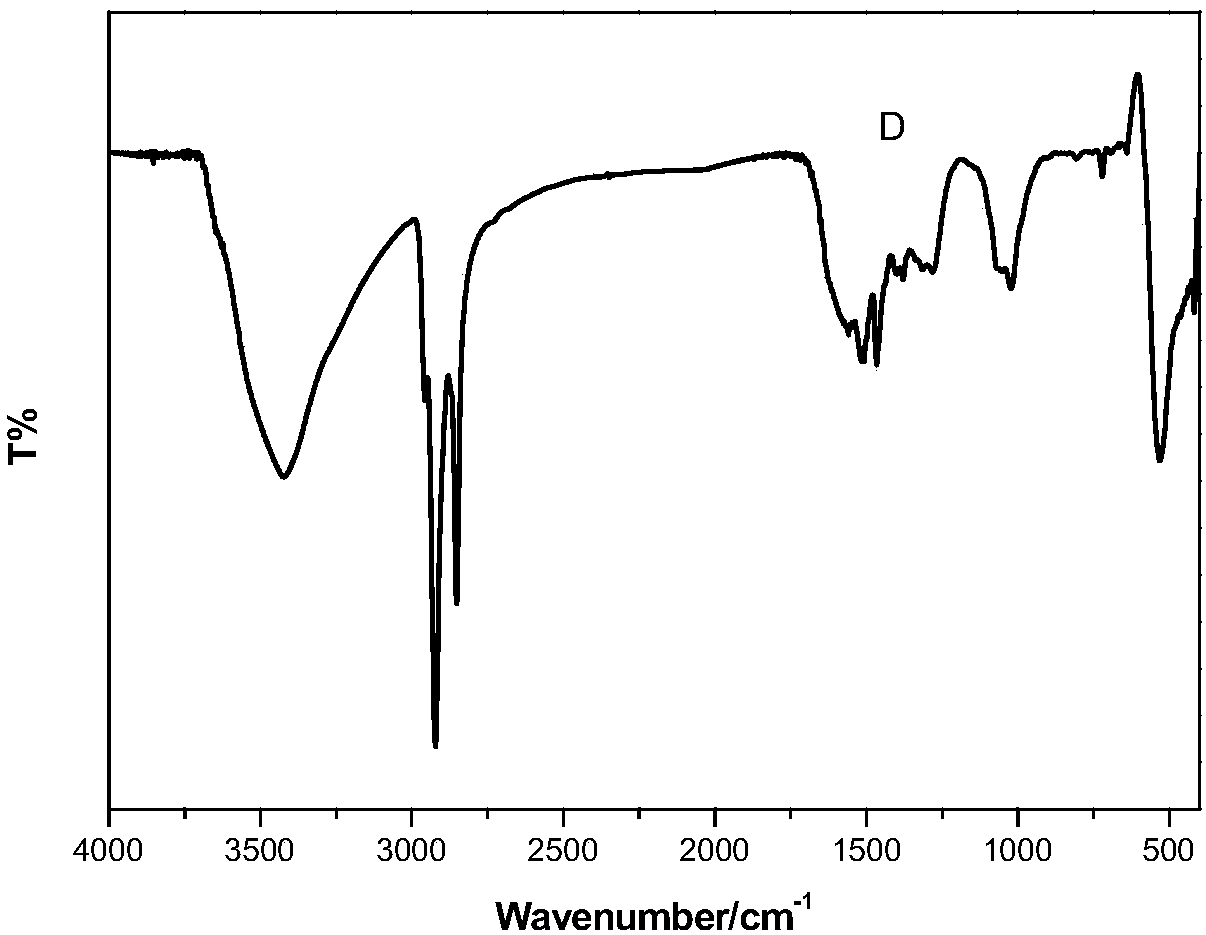
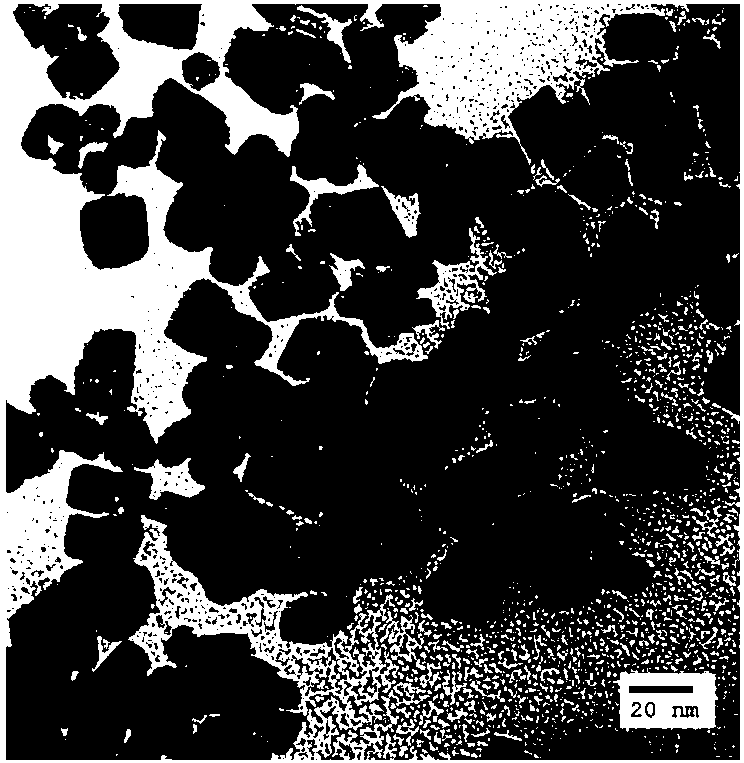
Catalytic selective oxidation method for hydrocarbons
A technology for selective oxidation and hydrocarbons, applied in organic chemical methods, chemical instruments and methods, oxidation reaction preparation, etc., can solve the problems of low catalytic efficiency, non-wetting, difficult contact between raw materials and catalysts, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of material A (HA-CoO)
[0028] 1) 9mmol CoCl 2 ·6H 2 O was dissolved in mixed solvent 1 (n-hexane 21ml, deionized water 12ml, ethanol 9ml), after stirring for 5min, 18mmol sodium oleate was added, and reacted at reflux temperature for 4h. After the reaction, wash with 30ml deionized water, separate the liquid, take the organic phase, and evaporate the solvent to obtain cobalt oleate;
[0029] 2) Take 1 mmol from the cobalt oleate obtained in step 1, and dissolve it in a mixed solvent 2 of octanol and n-hexylamine (HA), wherein 20 ml of octanol and 5 ml of n-hexylamine; stir at room temperature until the solid is completely dissolved;
[0030] 3) Transfer the uniformly mixed reaction solution to a 50ml autoclave, the reaction temperature is 250°C, and the reaction time is 5h;
[0031] 4) Cool down to room temperature naturally, collect the solid by centrifugation; wash with ethanol 20-30 times; collect the solid dried at room temperature to ...
Embodiment 2
[0032] Embodiment 2: the preparation of material B-1
[0033] The preparation method of material B-I is the same as material A, the difference lies in the type of organic amine and the metal salt used to prepare the precursor.
[0034] Table 1 Types of metal salts and organic amines used in material B-I
[0035]
[0036]
Embodiment 3
[0037] Embodiment 3: material J (CuMn 2 o 4 ) preparation
[0038] 1) 9mmol CuCl 2 ·6H 2 O was dissolved in mixed solvent 1 (n-hexane 21ml, deionized water 12ml, ethanol 9ml), stirred for 5min, added 18mmol sodium oleate, and reacted at reflux temperature for 4h. After the reaction, wash with 30ml of deionized water, separate the liquid, take the organic phase, and remove the solvent by rotary evaporation to obtain copper oleate;
[0039] 2) 9mmol MnCl 2 ·6H 2O was dissolved in mixed solvent 1 (n-hexane 21ml, deionized water 12ml, ethanol 9ml), stirred for 5min, added 18mmol sodium oleate, and reacted at reflux temperature for 4h. After the reaction, wash with 30ml deionized water, separate the liquid, take the organic phase, and remove the solvent by rotary evaporation to obtain manganese oleate;
[0040] 3) Take 0.5mmol from the copper oleate obtained in step 1, take 1mmol from the manganese oleate obtained in step 2, mix and dissolve in the mixed solvent 2 of octanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com