Inorganic electrolyte, preparation method thereof and lithium battery thereof
An electrolyte and inorganic technology, applied in electrolytes, secondary batteries, circuits, etc., can solve the problems that all-solid-state batteries do not have the conditions for industrialization, reduce the performance of organic electrolytes, and poor performance of all-solid-state batteries. Potential phenomenon, improving the electrochemical stability window, improving the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
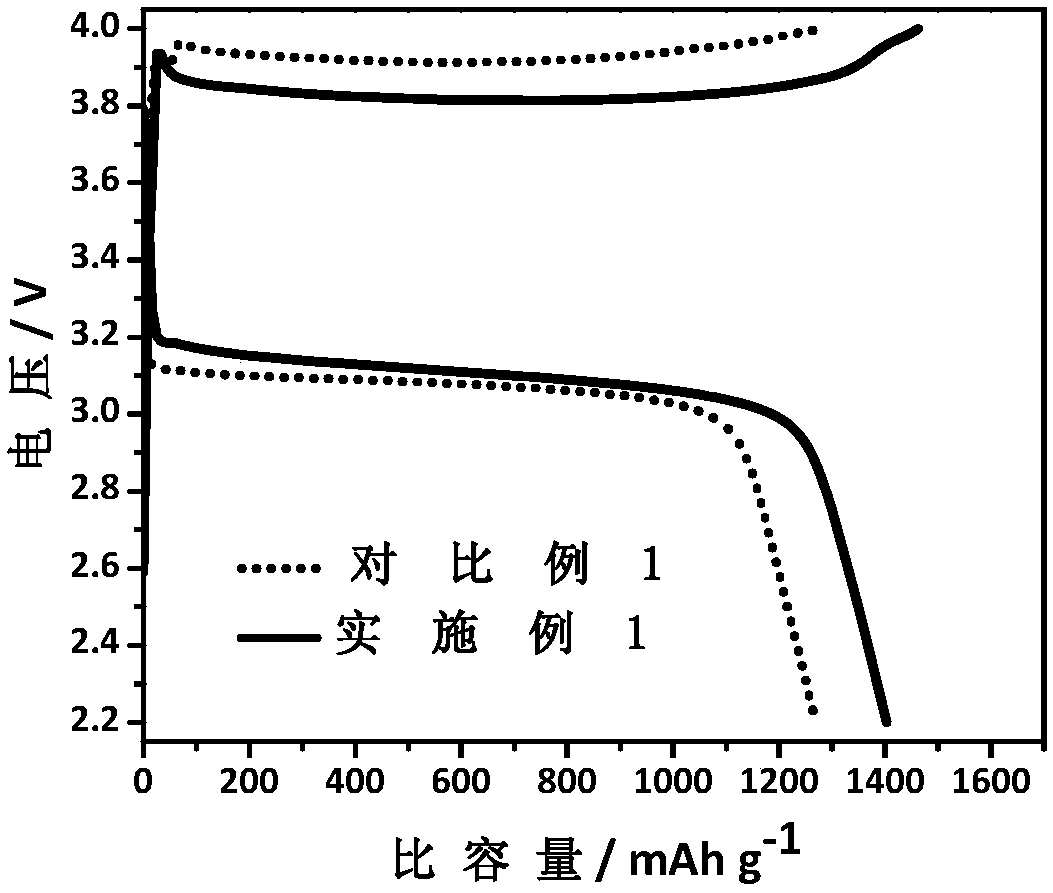
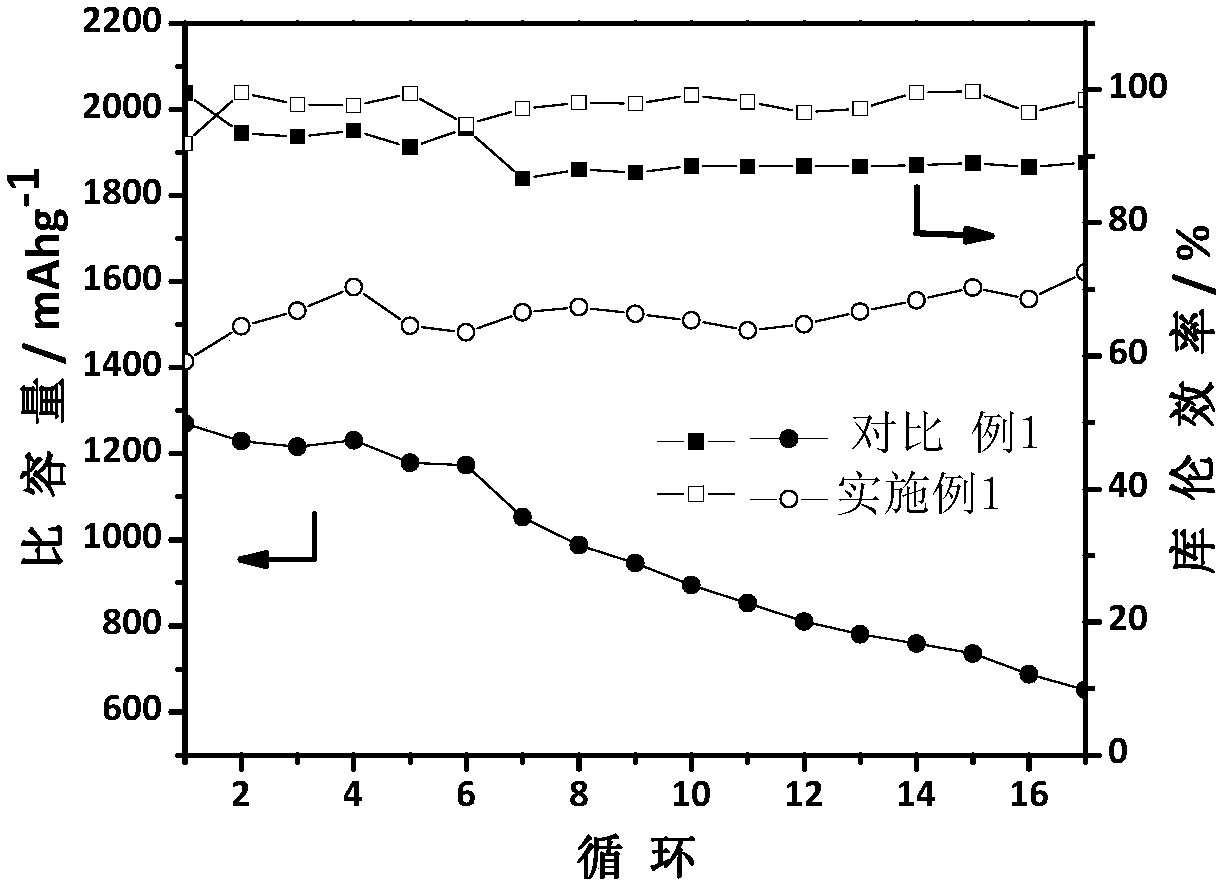
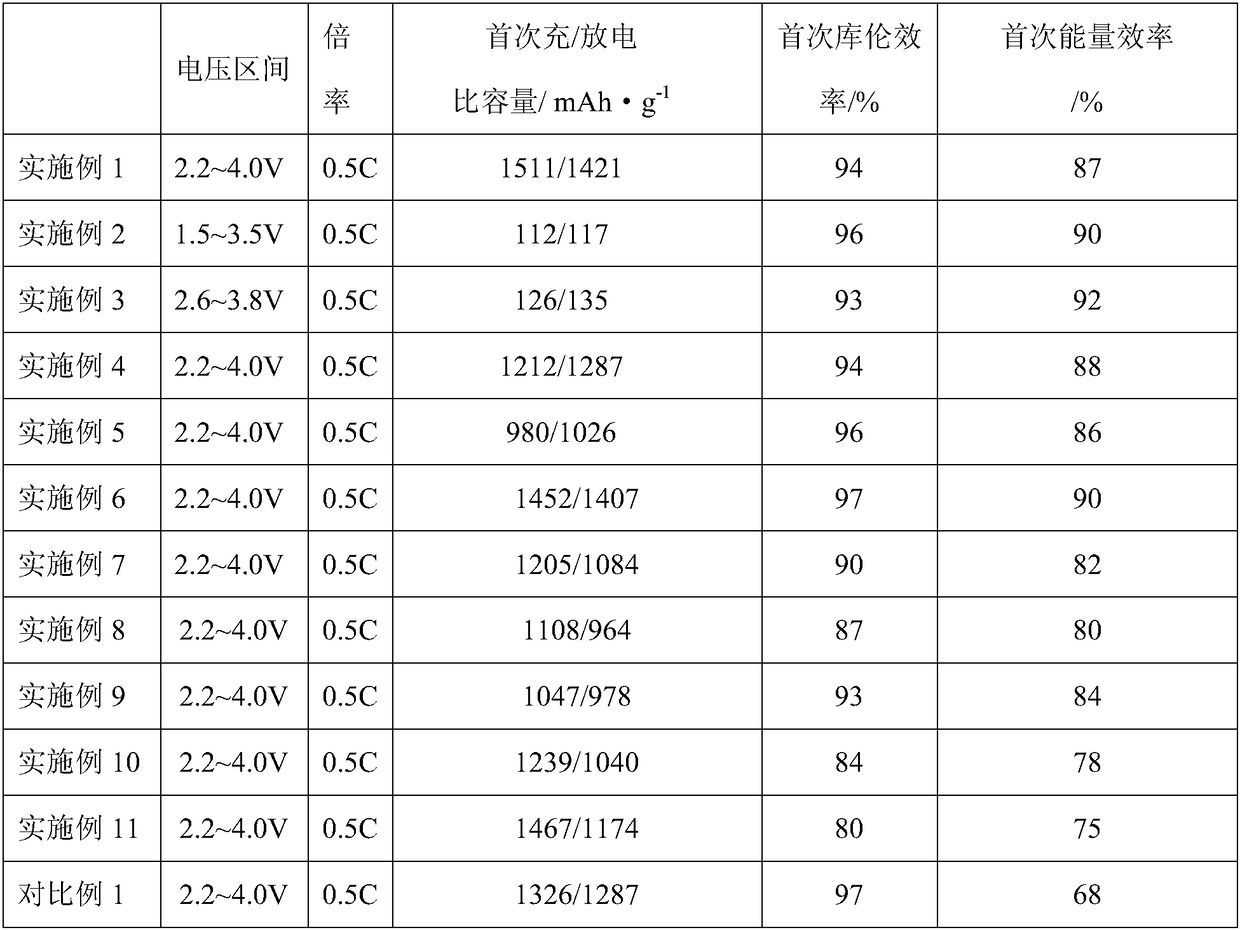
Examples
Embodiment 1
[0035] Embodiment 1: the first step: LiCl and AlCl 3 Mix evenly according to the stoichiometric ratio of 1.1:1, and dry in vacuum at 110°C for 3h.
[0036] The second step: the above LiCl and AlCl 3 The mixture is placed in a closed container and continuously fed with sulfur dioxide gas until liquid LiAlCl is obtained. 4 ·3SO 2 solution.
[0037] Step 3: Add the LiI inorganic salt additive to the above solution at a content of 2 wt%, and continue stirring and dissolving under the sulfur dioxide atmosphere.
[0038] The fourth step: to the above-mentioned LiAlCl containing LiI inorganic salt additive 4 ·3SO 2 Lithium metal flakes were added into the solution and allowed to stand for 24 hours to obtain an inorganic electrolyte.
[0039] Step 5: The positive electrode is made of Ketjen carbon, and the negative electrode is made of metal lithium sheet. The CR2032 button battery is assembled in an environment with controlled water and oxygen content, and the corresponding ele...
Embodiment 2
[0041] Embodiment 2: the first step: with NaCl and AlCl 3 Mix evenly according to the stoichiometric ratio of 1.1:1, and dry in vacuum at 120°C for 10h.
[0042] The second step: the above NaCl and AlCl 3 The mixture is placed in a closed container and continuously fed with sulfur dioxide gas until liquid LiAlCl is obtained. 4 ·2SO 2 solution.
[0043] The third step: the GaCl 3 and LiI inorganic salt additives were added to the above solution in the amount of 5wt% and 1wt%, respectively, and were continuously stirred and dissolved under the sulfur dioxide atmosphere.
[0044] Step 4: To the above-mentioned containing GaCl 3 LiAlCl and LiI inorganic salt additive 4 ·2SO 2 Sodium metal was added to the solution and left to stand for 24 hours to obtain an inorganic electrolyte.
[0045] Step 5: Lithium cobaltate is used for the positive electrode, and lithium titanate is used for the negative electrode. A CR2032 button battery is assembled in an environment with controll...
Embodiment 3
[0046] Embodiment 3: the first step: MgCl 2 and AlCl 3 Mix evenly according to the stoichiometric ratio of 1.1:2, and dry in vacuum at 150°C for 5 hours.
[0047] The second step: the above MgCl 2 and AlCl 3 The mixture is placed in a closed container and continuously fed with sulfur dioxide gas until liquid Mg (AlCl 4 ) 2 4.5SO 2 solution.
[0048] Step 3: Add the LiI inorganic salt additive into the above-mentioned liquid inorganic electrolyte at a content of 10 wt%, and continue stirring and dissolving under the sulfur dioxide atmosphere.
[0049] The fourth step: to the above-mentioned MgAlCl containing LiI inorganic salt additive 5 4.5SO 2 Lithium metal was added into the solution, and left to stand for 24 hours to obtain an inorganic electrolyte.
[0050] Step 5: Use lithium iron phosphate as the positive electrode and lithium metal sheet as the negative electrode. Assemble the CR2032 button battery in an environment with controlled water and oxygen content, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com