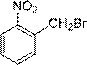Preparation method of nitrobenzyl bromide
A technology of o-nitrobenzyl bromide and o-nitrotoluene, which is applied in the field of preparation of o-nitrobenzyl bromide, can solve the problems of slow reaction rate and unsuitability for industrial production, and achieve the advantages of simple operation, high yield and low content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
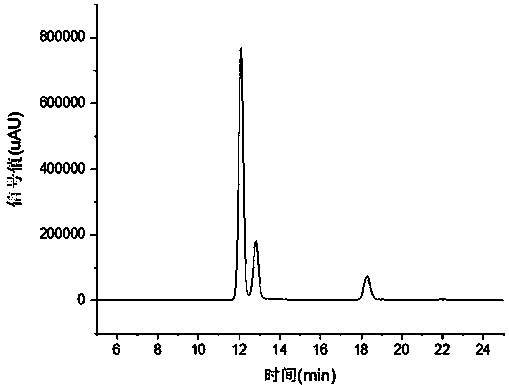
[0019] Weigh 6.85 grams of o-nitrotoluene and dissolve in 130 grams of dichloromethane, add 13.16 grams of 40% hydrogen bromide solution, 0.82 grams of azobisisobutyronitrile, and 0.81 grams of anhydrous ferric chloride to the system, and stir Heat to reflux, add 7.37 g of 30% hydrogen peroxide solution dropwise at 1 drop / min, stir and reflux for 2 hours, cool to room temperature, and separate the organic phase, which is o-nitrobenzyl bromide. As detected by HPLC, the yield was 86.31%, and the purity was 89.11%.
Embodiment 2
[0021] Weigh 6.85 grams of o-nitrotoluene and dissolve in 130 grams of dichloromethane, add 15.19 grams of 40% hydrogen bromide solution, 0.82 grams of azobisisobutyronitrile to the system, add 0.89 grams of cobalt acetate, stir and heat to Reflux, and dropwise add 8.50 g of 30% hydrogen peroxide solution at 2 drops / min, stir and reflux for 2 hours, cool to room temperature, and separate the organic phase, which is o-nitrobenzyl bromide. As detected by HPLC, the yield was 82.77%, and the purity was 91.11%.
Embodiment 3
[0023] Weigh 6.85 grams of o-nitrotoluene and dissolve in 130 grams of dichloromethane, add 17.21 grams of 40% hydrogen bromide solution, 0.82 grams of azobisisobutyronitrile to the system, add 0.68 grams of zinc chloride, stir and heat to reflux, and dropwise added 9.63 g of 30% hydrogen peroxide solution at 2 drops / min, stirred and refluxed for 2 hours, cooled to room temperature, and separated the organic phase, which was o-nitrobenzyl bromide. As detected by HPLC, the yield was 81.72%, and the purity was 93.83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com