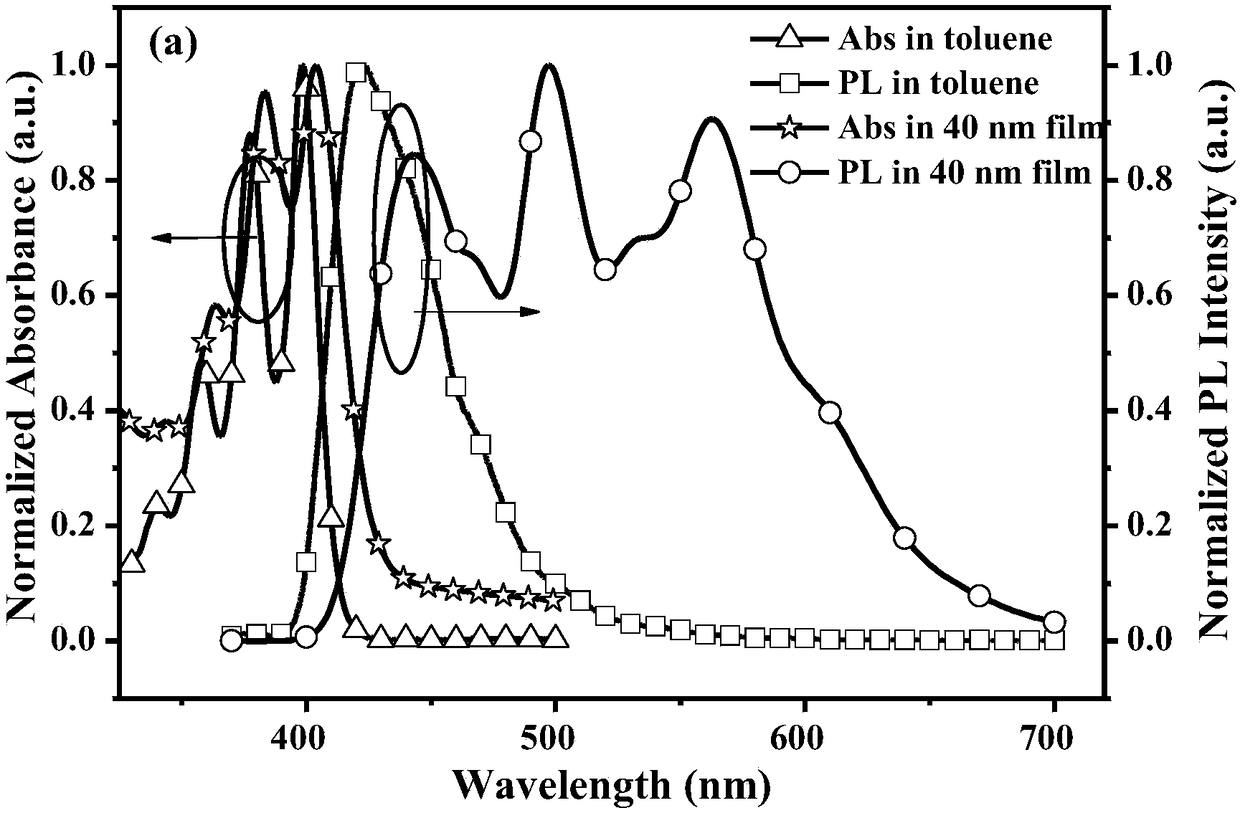
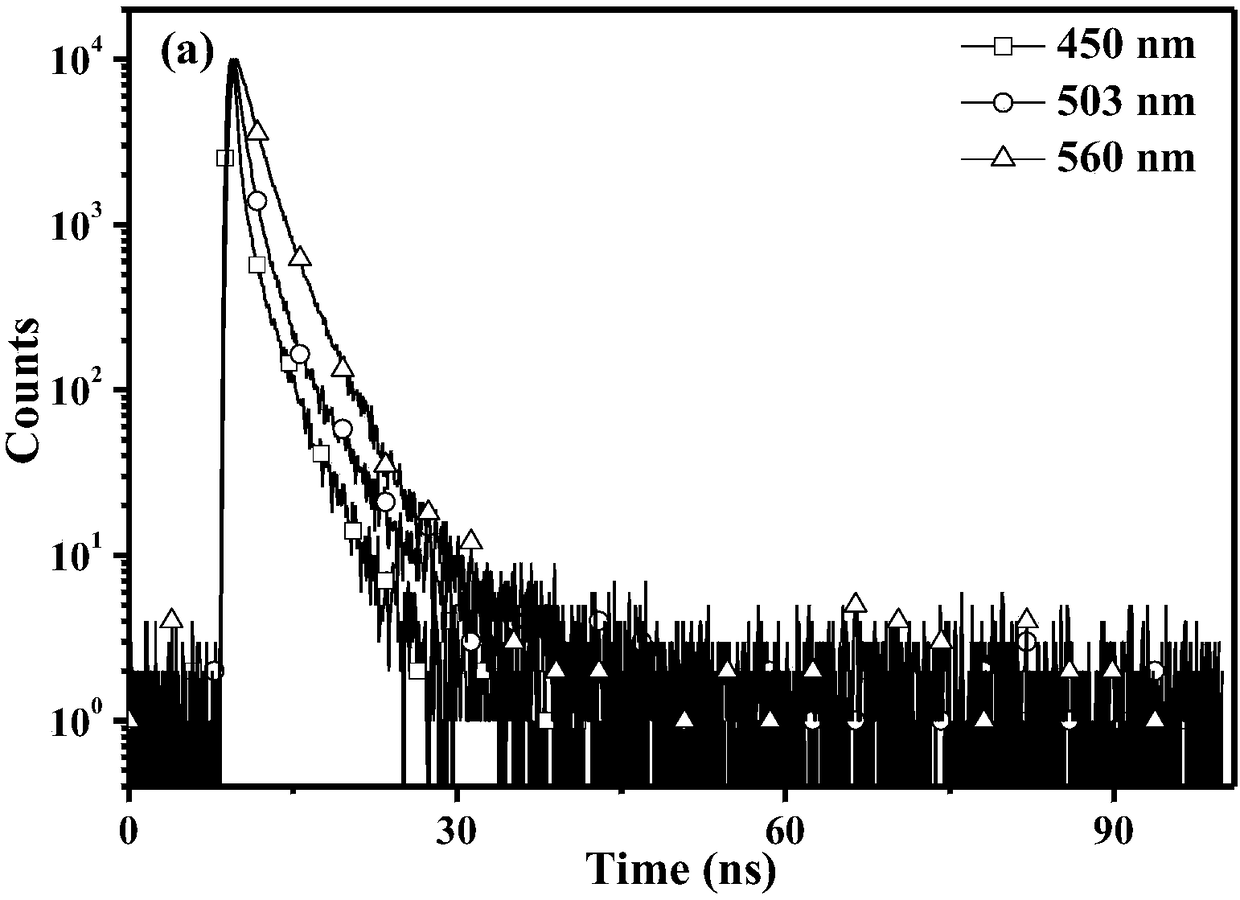
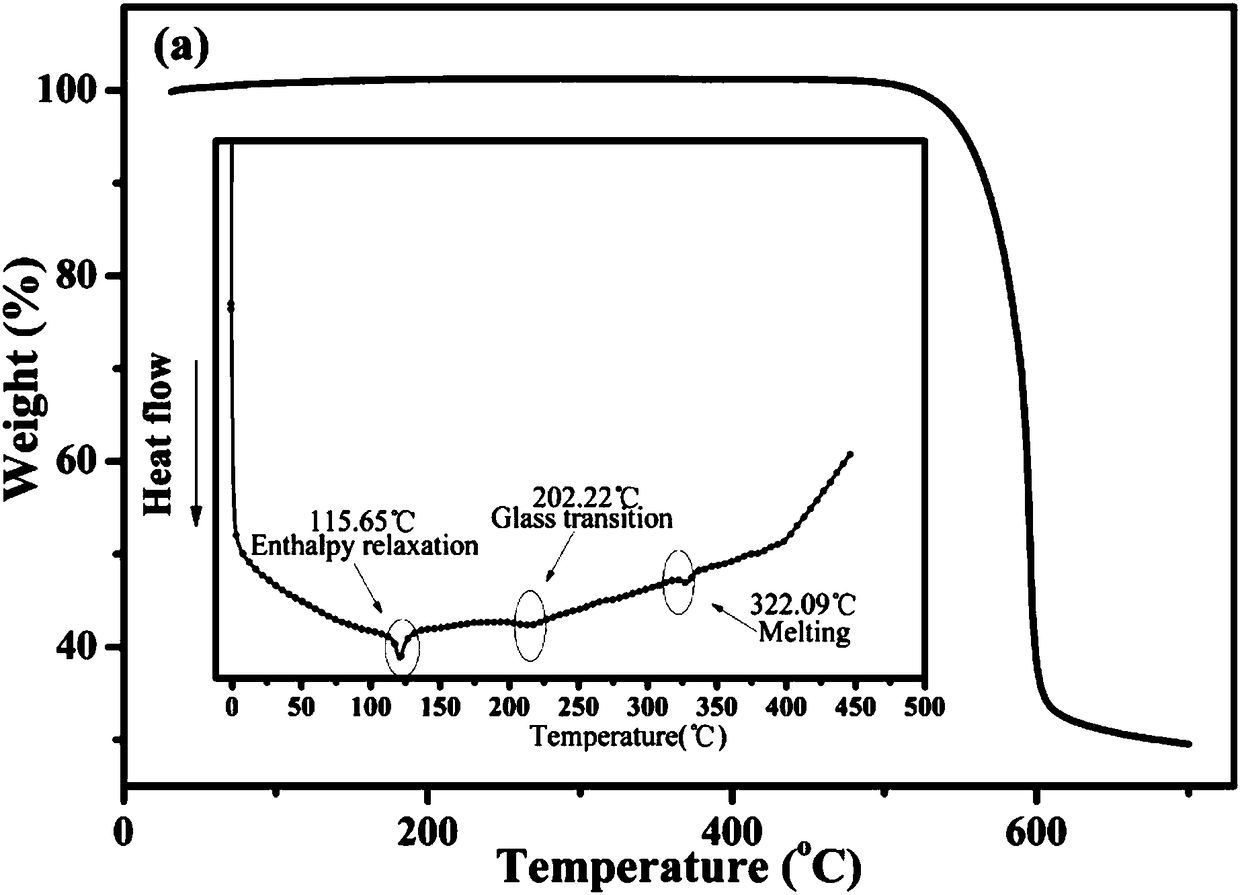
9-naphthanthracene derivative monomolecular white light material as well as preparation and application thereof
A derivative and single-molecule technology, applied in the field of 9-naphthalene anthracene derivative single-molecule white light materials and its preparation and application, can solve the problems of unstable light color and complicated preparation of single-molecule white light materials, and achieve low cost and stable The effect of white light emission and warm white light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The chemical reaction principles based on the preparation methods of these compounds can be summarized into three types:
[0043] The first:
[0044]
[0045] The second type:
[0046]
[0047] The third type:
[0048]
Embodiment 1
[0051] The structural formula of the 9-naphthalene anthracene derivative in this example is shown in formula 4, that is, 6,12-bis[10-(1-naphthyl)-9-anthracene)]-chrysene.
[0052]
[0053] The main chemical reactions that its preparation method takes place are as follows:
[0054]
[0055] Specifically: under nitrogen protection, 9-(1-naphthalene) anthracene-10-boronic acid (13mmol, 4.5g), 6,12-dibromochrome (5.4mmol, 2.1g), tetrakistriphenylphosphine palladium ( 0.3mmol, 0.4g), toluene (200mL), ethanol (100ml) and potassium carbonate aqueous solution (100mL, 2M) were put into a 500ml three-necked round bottom flask and stirred, and the mixture was heated to 90°C for 12 hours under the protection of nitrogen flow. After the reaction was completed, it was naturally cooled to room temperature, and suspended solids were precipitated. The precipitates were filtered out, washed with water, ethanol, and acetone respectively, placed in a vacuum drying oven for drying, and subli...
Embodiment 2
[0059] The structural formula of the 9-naphthalene anthracene derivative in this example is shown in formula 5, that is, 6,12-bis[10-(2-naphthyl)-9-anthracene)]-chrysene.
[0060]
[0061] Its preparation method is as follows: 9-(2-naphthalene) anthracene-10-boronic acid (13mmol, 4.5g), 6,12-dibromochrome (5.4mmol, 2.1g), tetrakis(triphenyl Phosphine) palladium (0.3mmol, 0.4g), toluene (200mL), ethanol (100ml) and potassium carbonate (100mL, 2M) were dropped into a 500ml three-necked round-bottomed flask and stirred, and the mixture was heated to 90°C under the protection of a nitrogen stream to react 12 Hour. After the reaction was completed, it was naturally cooled to room temperature, and suspended solids were precipitated. The precipitates were filtered out, washed with water, ethanol, and acetone respectively, placed in a vacuum drying oven for drying, and sublimated with a high-vacuum sublimator to obtain 3.9 g of a light yellow powder. The yield was : 87%.
[0062]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com