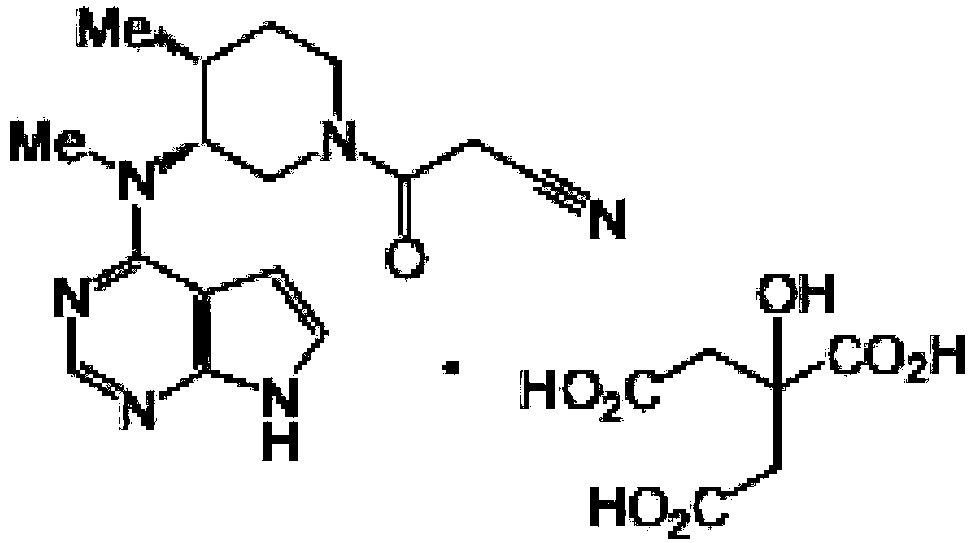
Tofacitinib citrate enteric-coated sustained-release pellet and preparation method thereof
A technology of sustained-release pellets and tofacitinib, which is applied in pharmaceutical formulations, microcapsules, medical preparations of non-active ingredients, etc., can solve problems such as difficult extrusion and difficult adjustment of release curves, and achieve stable release speed, The effect of good process reproducibility and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of tofacitinib citrate enteric-coated sustained-release pellets:
[0046] Prescription composition of tofacitinib citrate enteric-coated sustained-release pellets: specification 11mg (calculated as tofacitinib)
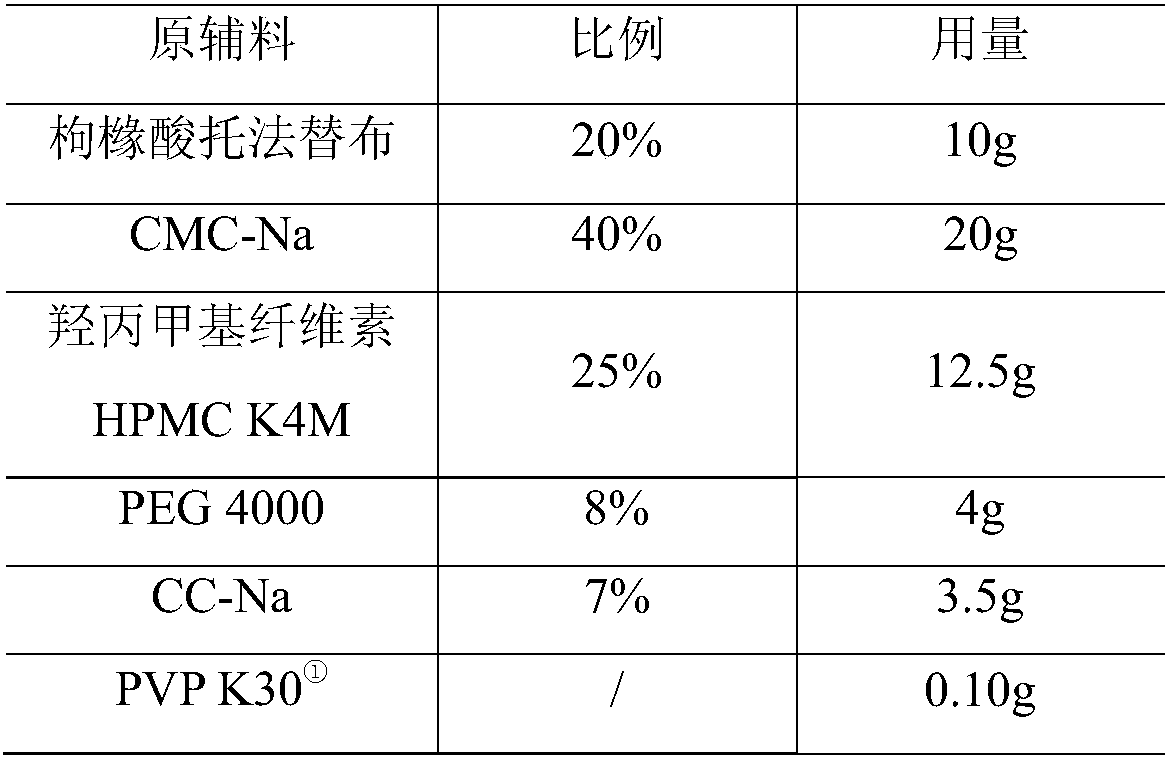
[0047] Ball core composition:
[0048]
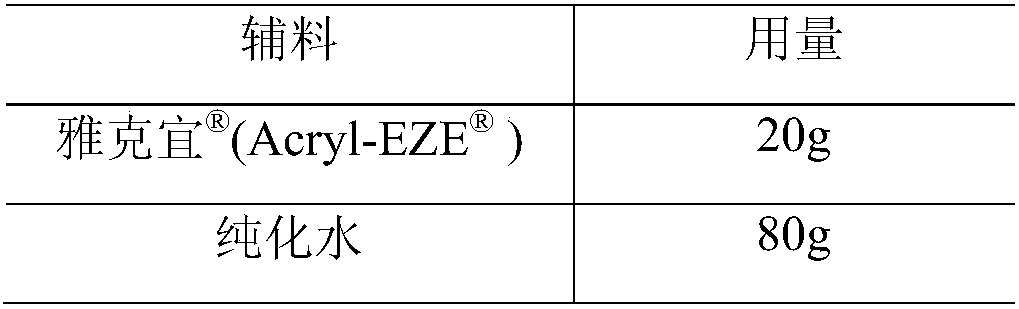
[0049] Coating liquid composition:
[0050]
[0051] Preparation Process:
[0052] (1) Weighing of raw and auxiliary materials
[0053] The raw and auxiliary materials were weighed in turn, and the purified water was weighed separately.
[0054] (2) Preparation of soft materials
[0055] ① Mix the weighed raw and auxiliary materials evenly according to the principle of equal increase.
[0056] ② Slowly add the binder under stirring, and stir until there is no lump-like material, and the over-wet soft material is obtained.
[0057] (3) Extrusion and spheronization to prepare pellets
[0058] Put the above-mentioned soft material in the extruder, adjust the speed of the extruder, then pl...
Embodiment 2
[0066] Example 2: Preparation of tofacitinib citrate enteric-coated sustained-release pellets:
[0067] Prescription composition of tofacitinib citrate enteric-coated sustained-release pellets: specification 11mg (calculated as tofacitinib)
[0068] Ball core composition:
[0069]
[0070] Coating liquid composition:
[0071]
[0072] Preparation process: with embodiment 1.
Embodiment 3
[0073] Example 3: Preparation of tofacitinib citrate enteric-coated sustained-release pellets:
[0074] Prescription composition of tofacitinib citrate enteric-coated sustained-release pellets: specification 11mg (calculated as tofacitinib)
[0075] Ball core composition:
[0076]
[0077] Coating liquid composition:
[0078]
[0079] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com