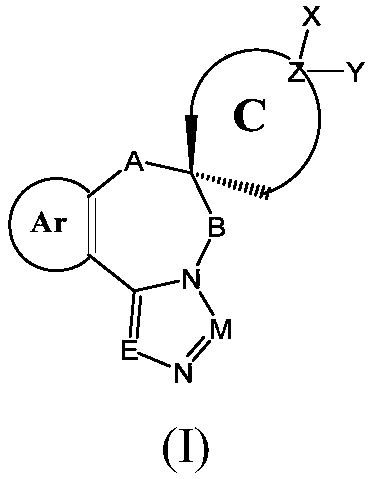
Efficient IDO/TDO double inhibitor containing nitrogen heterocyclic helix structure
A cycloalkyl and heteroaryl technology, applied in the field of high-efficiency IDO/TDO inhibitors and its preparation, can solve problems such as cell dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0253] Preparation of (cis / trans) 3-phenylspiro[cyclobutane 1,5'-imidazo[5,1-α]isoindole]-3-ol
[0254]
[0255] The first step: (cis / trans) 1-(2-bromophenyl)-3-hydroxycyclobutanenitrile
[0256]
[0257] Add the compound 2-bromobenzylcyanide (10.2g, 52.0mmol) into the reaction flask, cool to minus 78 degrees, add methyllithium (44mL, 52.0mmol) dropwise, after the dropwise addition, stir at this temperature for 1 hour, add Epichlorohydrin (5.3g, 57.2mmol), stirred at this temperature for 3 hours, TLC detected that the raw material disappeared, then added dropwise methylmagnesium bromide Grignard reagent (19ml, 57.2mmol), after the dropwise addition, naturally returned to room temperature , and then slowly warming up to 60 degrees, stirring overnight. TLC monitors that the reaction is complete. Under cooling in an ice bath, 2N hydrochloric acid solution was added dropwise, and the pH of the reaction system was adjusted to about 7. Ethyl acetate (150 mL) and water (150 ...
Embodiment 2
[0294] (cis / trans)(3-fluorophenyl)(spiro[cyclobutane 1,5'-imidazo[5,1-α]isoindol]-3-yl)methanol
[0295]
[0296] Step 1: (cis)spiro[cyclobutane 1,5'-imidazo[5,1-α]isoindole]-3-yl methanesulfonate
[0297]
[0298] (cis)spiro[cyclobutane 1,5'-imidazo[5,1-α]isoindole]-3-ol (212mg, 1.0mmol) was dissolved in 5mL of dichloromethane, triethylamine ( 202mg, 2.0mmol), methanesulfonyl chloride (216mg, 1.5mmol) was added dropwise, after the dropwise completion, it was naturally returned to room temperature, and reacted for 1 hour in total. Add saturated brine 10mL, respectively add dichloromethane 2x 20mL for extraction, combine the organic phases to obtain the target compound (300mg, yield 100%).
[0299] MS ESI: m / z=291.0, [M+H] + .
[0300] The second step: spiro[cyclobutane 1,5'-imidazo[5,1-α]is indole]-3-carbonitrile
[0301]
[0302] The product of the first step (300mg, 1.0mmol) was dissolved in 10mL of DMF, sodium cyanide (150mg, 3.0mmol) was added, heated to 100°C...
Embodiment 3
[0324] (cis / trans) 3-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)spiro[cyclobutane 1,5'-imidazo[5,1-α]isoind Indole]-3-ol
[0325]
[0326] According to the conditions in the eighth step of Example 1, the target compound was obtained as a white solid (10 mg, Yield 29%).
[0327] 1 H NMR (400MHz, CDCl 3 ):δ8.35(s,1H),7.42-7.41(m,1H),7.37-7.35(m,1H),7.34-7.31(m,1H),7.27-7.25(m,1H),7.18(s ,1H),5.75(s,1H),3.86(s,4H),3.15(d,2H),2.64(d,2H),2.40-2.36(m,4H),1.83-1.80(m,2H).
[0328] MS ESI: m / z=351.2, [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com