Method for preparation of tert-amyl alcohol by isoamylene hydration
A technology of isopentene water and tert-amyl alcohol, applied in hydroxyl addition preparation, organic chemistry and other directions, can solve problems such as large energy consumption and high production cost, achieve low energy consumption, save energy consumption, and save production and operation costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
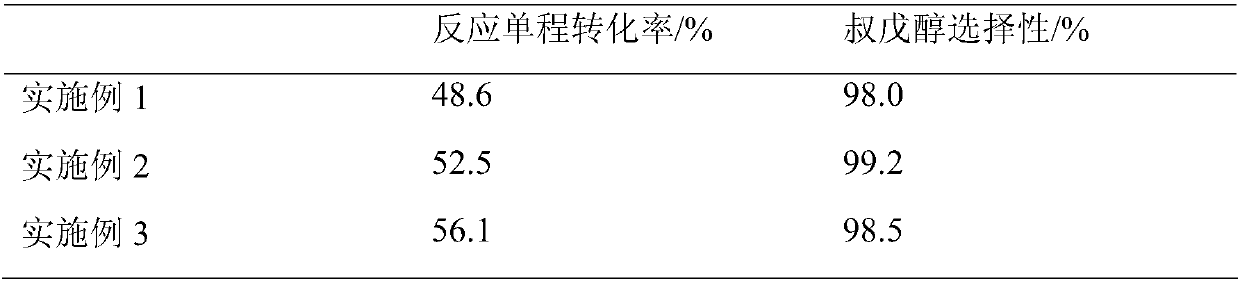
Embodiment 1
[0019] After mixing the isoamylene component rich in 2-methyl-1-butene and 2-methyl-2-butene with water and acetone, it enters the fixed-bed reaction equipped with strong acidic cation exchange resin catalyst The hydration reaction is carried out in a container to obtain tert-amyl alcohol. Wherein: the mass ratio of water, acetone and isoamylene rich in 2-methyl-1-butene and 2-methyl-2-butene in the hydration reaction raw material to acetone is 1:39.0:4.0. During the hydration reaction, the liquid hourly volume space velocity is 1.0h -1 , the reaction temperature is 50°C, and the reaction pressure is 1MPa.
Embodiment 2
[0021] After mixing the isoamylene component rich in 2-methyl-1-butene and 2-methyl-2-butene with water and acetone, it enters the fixed-bed reaction equipped with strong acidic cation exchange resin catalyst The hydration reaction is carried out in a container to obtain tert-amyl alcohol. Wherein: the mass ratio of water, acetone and isoamylene rich in 2-methyl-1-butene and 2-methyl-2-butene in the hydration reaction raw material to acetone is 1:2.0:6.0. During the hydration reaction, the liquid hourly volume space velocity is 2.0h -1 , the reaction temperature is 60°C, and the reaction pressure is 0.5MPa.
Embodiment 3
[0023] After mixing the isoamylene component rich in 2-methyl-1-butene and 2-methyl-2-butene with water and acetone, it enters the fixed-bed reaction equipped with strong acidic cation exchange resin catalyst The hydration reaction is carried out in a container to obtain tert-amyl alcohol. Wherein: the mass ratio of water, acetone and isoamylene rich in 2-methyl-1-butene and 2-methyl-2-butene in the hydration reaction raw material to acetone is 1:13.0:13.0. During the hydration reaction, the liquid hourly volume space velocity is 0.1h -1 , the reaction temperature is 70°C, and the reaction pressure is 1.5MPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com