Method for preparing erivedge by adopting microchannel reaction device
A technology of microchannel reaction and microstructure reactor, which is applied in the direction of organic chemistry, can solve the problems of high cost, complex reaction conditions, and long reaction time, and achieve the effects of high continuity, simple operation, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
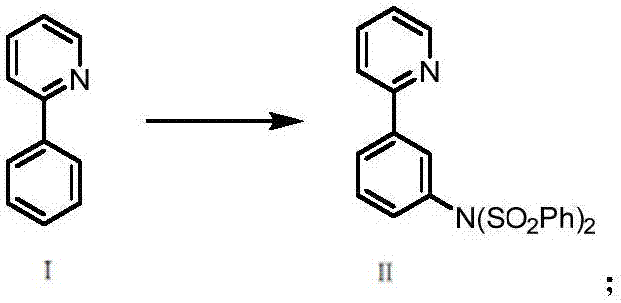
[0033] (1) Preparation of N-(phenylsulfonyl)-N-)3-(pyridin-2-yl)phenyl)benzenesulfonamide (compound Ⅱ):
[0034]
[0035] Add 10mmol 2-phenylpyridine, 15mmol diphenylsulfonimide, 0.5mmol triruthenium dodecacarbonyl, 20mmol iodobenzene diacetate, 40mL dichloroethane to a 100mL reaction flask, that is, compound Ⅰ, diphenylsulfonyl The molar ratio of imine, metal catalyst (triruthenium dodecacarbonyl) and oxidant (iodobenzene diacetate) is: 1:1.5:0.05:2, reflux in an oil bath at 100°C for 24 hours, cool down to room temperature, filter, and recover Triruthenium dodecacarbonyl, then add 100 mL of water to the filtrate, then add dichloromethane to extract three times (100 mL×3), combine the organic phases, wash with saturated brine, add anhydrous sodium sulfate to dry, and distill off the solvent under reduced pressure, Column chromatography (mobile phase V / V: ethyl acetate / petroleum ether=1 / 4) gave a white solid with a yield of 80%; HRMS (ESI-TOF-MS) m / z: 451.0776 ([M+H] + ); ...
Embodiment 2
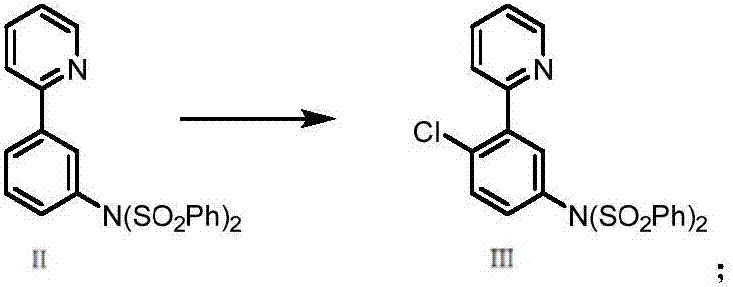
[0045] The preparation method is the same as in Example 1, but the reaction temperature of step (1) is 125° C. to obtain white N-(phenylsulfonyl)-N-(3-(pyridin-2-yl)phenyl)benzenesulfonamide (compound Ⅱ), yield 75%;
[0046] Step (2) The reaction temperature is 120°C, and white N-(4-chloro-3-(pyridin-2-yl)phenyl)-N-(phenylsulfonyl)benzenesulfonamide (compound III) is obtained in a yield of 82%;
[0047] Step (3) The reaction temperature was 50°C, and 4-chloro-3-(pyridin-2-yl)aniline (compound IV) was obtained as a colorless oil with a yield of 94%;
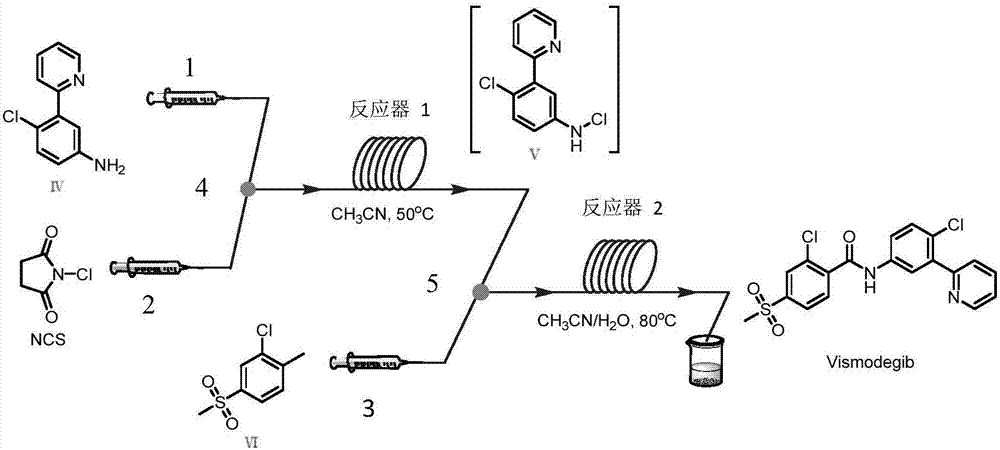
[0048] Step (4) The reaction temperature of the first microstructure reactor is 100° C., and the reaction temperature is 100° C., and the residence time is 5 minutes. The reaction temperature of the second microstructure reactor is 120° C., and the residence time is 10 minutes to obtain white Vimodergib with a yield of 85%.
Embodiment 3
[0050] The preparation method is the same as in Example 1, but the reaction temperature of step (1) is 25° C. to obtain white N-(phenylsulfonyl)-N-(3-(pyridin-2-yl)phenyl)benzenesulfonamide (compound Ⅱ), yield 65%;
[0051] Step (2) The reaction temperature is 80°C, and white N-(4-chloro-3-(pyridin-2-yl)phenyl)-N-(phenylsulfonyl)benzenesulfonamide (compound III) is obtained. The yield is 83%;
[0052] Step (3) The reaction temperature was 0°C, and 4-chloro-3-(pyridin-2-yl)aniline (compound IV) was obtained as a colorless oil with a yield of 90%;
[0053] Step (4) The reaction temperature of the first microstructure reactor is 25° C., and the residence time is 20 minutes. The reaction temperature of the second microstructure reactor is 50° C., and the residence time is 50 minutes to obtain white Vimodeji with a yield of 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com