peg-modified water-soluble prodrugs of triacontanol
A triacontanol, water-soluble technology, applied in the field of water-soluble triacontanol macromolecular prodrugs, can solve the problem of poor water solubility of triacontanol and low attention, and achieves improved water solubility, increased yield, improved water solubility, and improved water solubility. The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0087] Example 1 Preparation of PEG-modified triacontanol linked by amide-ester linkage
[0088] (1) Preparation of compound a
[0089] Prepare 10mmol mPEG 2k Dissolved in toluene and dried by azeotropic distillation to remove the toluene, the solution was first warmed and then cooled in an ice-water bath (under nitrogen atmosphere). A mixture of pyridine (20 mmol) and methanesulfonyl chloride (80 mmol) was added and reacted overnight under nitrogen. The solution was filtered under vacuum until clear, precipitated with ether, filtered, and dried in vacuo. The resulting product was mixed with potassium phthalimide, added with 100 mL of DMF, placed in nitrogen, and stirred at 120°C for 4 hours. The precipitate was filtered off and dissolved in 200 mL of ethanol, then 10 mL of hydrazine hydrate was added. The mixture was heated to reflux for 4 hours. After cooling to room temperature, the product was precipitated by adding diethyl ether dropwise to the solution. The precipi...
example 2
[0093] Add compound b (0.5g), triacontanol (0.5g), EDCI (1mol), DIPEA (3mol), DMAP (0.05g), 20ml of dichloromethane into a 100ml one-necked bottle, and stir overnight at room temperature. The next day, add 50ml of dichloromethane to the reaction flask, wash twice, dry the organic phase with anhydrous sodium sulfate, and perform column chromatography (developing solvent is dichloromethane:methanol 15:1), to obtain compound c (see figure 1 )0.51g, yield 82.6%. Example 2 Preparation of PEG-modified triacontanol with succinate as the linkage
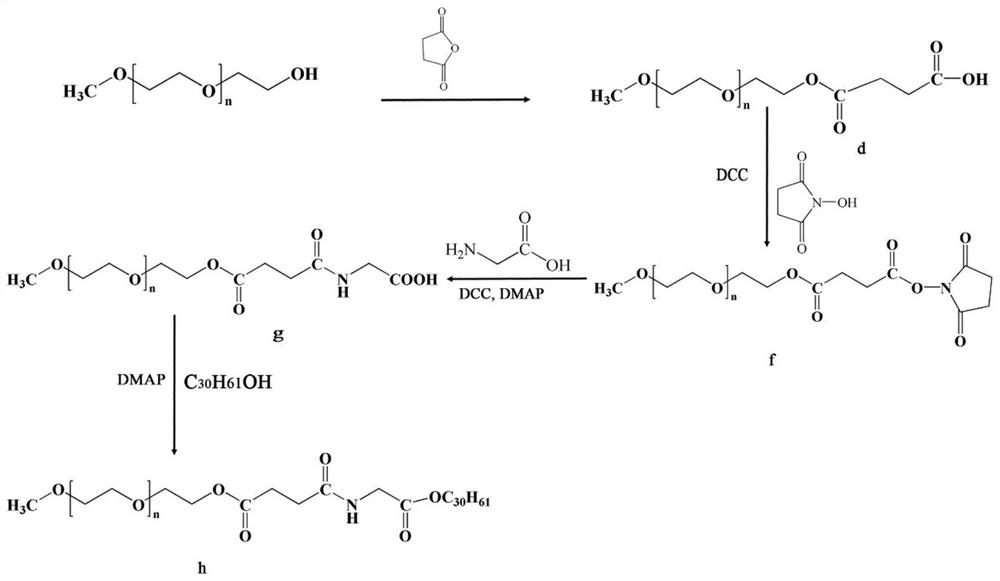
[0094] (1) Preparation of compound d
[0095] mPEG 2k5 mmol was dissolved in toluene and dried by azeotropic distillation, then 25 mmol of succinic anhydride, DMAP (10 mmol) and triethylamine (10 mmol) were added, and the mixture was stirred overnight at room temperature. After the reaction was complete, the mixture was cooled and placed in CH 2 Cl 2 , and precipitate the polymer with ether. The precipitate was filtered, washed, and dr...
example 3
[0098] Example 3 Preparation of PEG-modified Triacontanol Linked to Glycine
[0099] (1) Preparation of compound f
[0100] Compound d 5mmol, DCC 10mmol and hydroxysuccinimide 10mmol, DMAP 5mmol were added into dichloromethane, and then the reaction mixture was stirred at room temperature for 24h. Dichloromethane was added to the mixture for multiple extractions, and the organic phases were combined, concentrated, and ether was added for precipitation. The precipitate was filtered, washed, dried by adding anhydrous sodium sulfate, and the residue was recrystallized with anhydrous ether to obtain active ester compound f.
[0101] (2) Preparation of compound g
[0102] Dissolve 5mmol of compound f in DMF, after the compound is completely dissolved, add 10mmol of glycine NaHCO 3 solution, then the reaction mixture was stirred overnight at room temperature, filtered and evaporated under reduced pressure, dichloromethane and water were added to the concentrate, the pH was adjust...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com