Monoclonal antibody 9A and application thereof
A technology combining molecules and heavy chains, applied in the fields of cellular immunology and molecular biology, can solve problems such as toxic side effects and poor specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1 Antibody Screening
[0088] In order to avoid the bias of individual immune background and ensure the diversity of the antibody library as much as possible, the peripheral blood of 100 healthy adults (half male and female) and umbilical cord blood lymphocytes of 10 neonates (half male and female) were separated by lymphocyte separation medium , a total of 2×10 9 cells. Total RNA was extracted by Trizol method, reverse transcribed into cDNA, and the variable region genes of different antibody subtypes were amplified by conventional PCR. According to the information of the antibody library vector pDF (Journal of the Academy of Military Medical Sciences, 2008, 32 (4): 305-308, 358.), the restriction site BssH II, Nhe I and the Loxp511 sequence (sequence: SGGSTITSYNVYYTKLSSSGT (SEQ The connecting peptide of ID NO: 15)) was spliced into ScFv (single-chain antibody: VL-Linker (containing Loxp511 sequence)-VH, BssH II and Nhe I sites were introduced into the upst...
Embodiment 2
[0091] Example 2 Antibody expression and purification
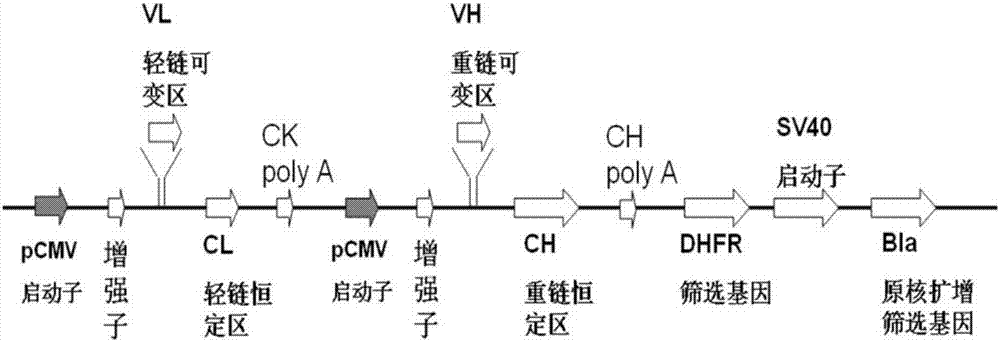
[0092] The obtained 9A clone variable region gene was cloned into the eukaryotic expression vector pCMV-163 containing the human IgG constant region gene to construct a full antibody expression vector, and its physical map is as follows: figure 1Shown (each component of the eukaryotic expression vector pCMV-163 is a component known in the art, recombined according to the sequence shown). The whole antibody is called 9A antibody (the light chain amino acid sequence is SEQ ID NO: 11, the nucleotide sequence is SEQ ID NO: 12; the heavy chain amino acid sequence is SEQ ID NO: 13, the nucleotide sequence is SEQ ID NO: 14) . The obtained eukaryotic expression vector was transfected into CHO-S cells by using the ExpiCHOTM Expression System kit (Thermo Fisher Scientific, #A29133), and the goat anti-human IgG (KPL, KPL, #01-10-06) and horseradish enzyme-labeled goat anti-human IgG (GOAT Antihuman (HRP), Thermo Fisher Scientific,...
Embodiment 3
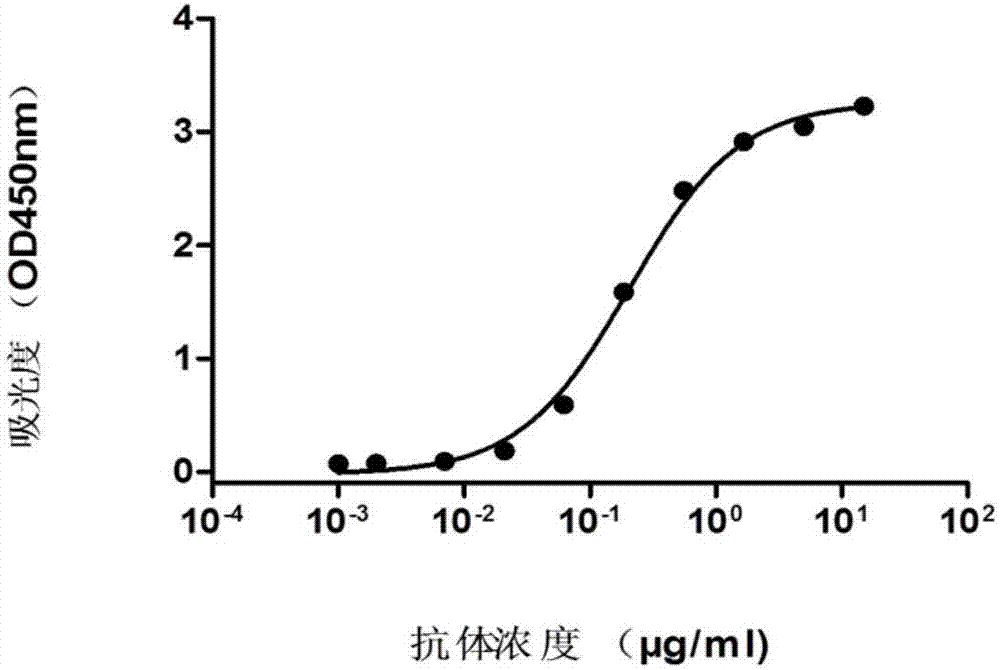
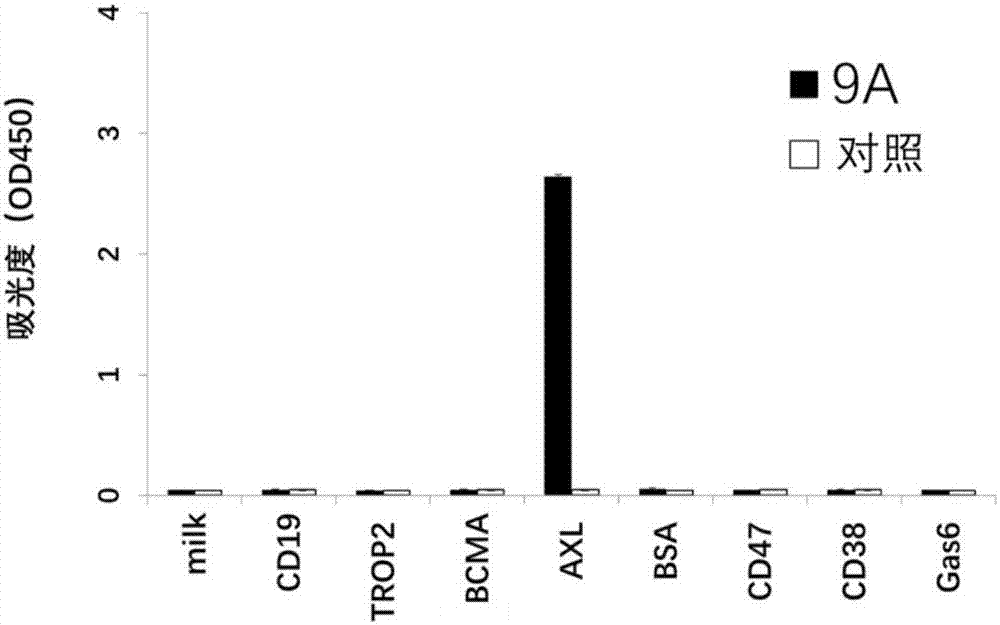
[0093] Example 3 Antibody Binding Activity Analysis
[0094] Coat the target antigen AXL-Fc on the ELISA plate, 1 μg / ml, overnight at 4°C; after washing with PBST, add 10% fetal bovine serum, block at 37°C for 1 hour; add different concentrations of 9A antibody, react at 37°C for 1 hour ; After washing with PBST, add horseradish peroxidase-labeled goat anti-human Fab secondary antibody (Goat Anti-human IgG (Fab') 2-HRP, Abcam), and react at 37°C for 30 minutes; repeat washing the plate 5 times with PBST, Pat dry the remaining droplets on absorbent paper as much as possible; add 100 μl TMB (eBioscience) to each well, and place in the dark at room temperature (20±5°C) for 1.5 min; add 100 μl 2N H to each well 2 SO 4 The stop solution terminated the substrate reaction, and the OD value was read at 450nm on a microplate reader to analyze the binding ability of the antibody to the target antigen AXL-Fc. The 9A antibody can specifically recognize the target antigen AXL-Fc; the rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com