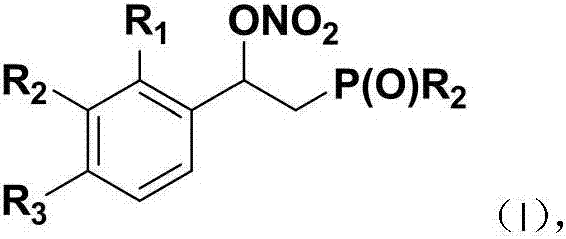
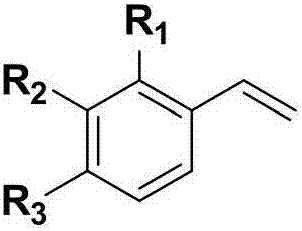
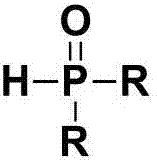
Beta-phosphoryl nitrate ester compounds and preparation method thereof
A technology of diphenylphosphoethyl nitrate and compound, which is applied in the field of β-phosphorylated nitrate compound and its preparation, can solve the problems of poor substrate compatibility, poor atom economy, and limited application, and achieve substrate Good compatibility, high conversion rate, and various effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Using styrene and diphenylphosphine oxide as raw materials, the reaction formula and experimental steps are as follows:
[0043]
[0044] Add diphenylphosphine oxide (40.4mg, 0.2mmol) and ammonium cerium nitrate (219mg, 0.4mmol) into a Schlenk tube equipped with magnetic stirring, and use double row tubes to replace nitrogen repeatedly, so that the whole system is under nitrogen atmosphere , and then added styrene (52mg, 0.5mmol) and 2mL 1,4-dioxane to the system, the reaction was carried out at 40°C, and the reaction was tracked by TLC until the end of the reaction. After the reaction was finished, the reaction solution was concentrated, and the mixed solution of petroleum ether and ethyl acetate with a volume ratio of 3:1 was used as an eluent, and the product 2-diphenylphosphine-1-phenylethyl nitrate was obtained by column chromatography. The rate is 88%.
[0045] 1 H NMR (400MHz, CDCl 3 ): δ7.74-7.79(m, 2H), 7.42-7.60(m, 6H), 7.29-7.37(m, 4H), 7.23-7.27(m, 3H)...
Embodiment 2
[0047] With 4-fluorostyrene and diphenylphosphine oxide as raw materials, the reaction formula and experimental steps are as follows:
[0048]
[0049] Add diphenylphosphine oxide (40.4mg, 0.2mmol) and ammonium cerium nitrate (219mg, 0.4mmol) into a Schlenk tube equipped with magnetic stirring, and use double row tubes to replace nitrogen repeatedly, so that the whole system is under nitrogen atmosphere , and then added 4-fluorostyrene (61mg, 0.5mmol) and 2mL 1,4-dioxane to the system, the reaction was carried out at 40°C, and the reaction was followed by TLC until the end of the reaction. After the reaction, the reaction solution was concentrated, and the mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 was used as the eluent, and the product 1-(4-fluorophenyl)-2-diphenylphosphine was separated by column chromatography Ethyl nitrate, 84% yield.
[0050] 1 H NMR (400MHz, CDCl 3 ): δ7.72-7.77(m, 2H), 7.43-7.58(m, 6H), 7.27-7.37(m, 4H), 6.86-6.92(m, ...
Embodiment 3
[0052] With 4-chlorostyrene and diphenyl phosphorus oxide as raw materials, the reaction formula and experimental steps are as follows:
[0053]
[0054] Add diphenylphosphine oxide (40.4mg, 0.2mmol) and ammonium cerium nitrate (219mg, 0.4mmol) into a Schlenk tube equipped with magnetic stirring, and use double row tubes to replace nitrogen repeatedly, so that the whole system is under nitrogen atmosphere , and then added 4-chlorostyrene (27.6mg, 0.5mmol) and 2mL 1,4-dioxane to the system, the reaction was carried out at 40°C, and the reaction was tracked by TLC until the end of the reaction. After the reaction, the reaction solution was concentrated, and the mixture of petroleum ether and ethyl acetate with a volume ratio of 3:1 was used as the eluent, and the product 1-(4-chlorophenyl)-2-diphenylphosphine was separated by column chromatography Ethyl nitrate, 78% yield.
[0055] 1 H NMR (400MHz, CDCl 3 ): δ7.72-7.77(m, 2H), 7.44-7.58(m, 6H), 7.32-7.37(m, 2H), 7.22-7.25(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com