A kind of dihydronaphthalene derivative substituted by difluoromethyl and its synthetic method
The technology of a difluoromethyl group and a synthetic method is applied in the field of organic chemical synthesis, and can solve the problems of expensive fluorination reagents, difficult synthesis, harsh reaction conditions, etc., and achieves good substrate universality, simple operation, and simple operation of reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] This embodiment provides a method for synthesizing dihydronaphthalene derivatives substituted with difluoromethyl, and then introducing fluorine atoms into the dihydronaphthalene derivatives. The method specifically includes the following steps:
[0049] After the reactor is evacuated, it is replaced by an inert gas such as nitrogen or argon, and then the methylenecyclopropane compound, brominated difluorocarbonyl compound, copper catalyst, ligand, pinacol diborate are added to the reactor in sequence Esters, bases and solvents are stirred and reacted at a temperature of 40-120°C;
[0050] After the reaction, the solvent was removed with a rotary evaporator to obtain a crude product, which was obtained through column chromatography, wherein the eluent used in the column chromatography was a mixed solvent of petroleum ether and ethyl acetate.
[0051] Above-mentioned copper catalyst is CuCl, CuBr, CuI, CuCl 2 、CuBr 2 and Cu(OAc) 2 One or more of them; the ligands are ...
Embodiment 1
[0068]
[0069] After the reactor was evacuated, the inert gas nitrogen or argon was replaced, and 0.2mmol (41.2mg) 1a, 0.4mmol (80.8mg) ethyl difluorobromoacetate (2a), 0.02mmol (2.9mg) CuBr, 0.02 mmol (7.1 mg) dtbbpy, 0.06 mmol (15.2 mg) B 2 pin 2 , 0.4 mmol (33.6 mg) NaHCO 3 , 1mL 1,4-dioxane, stirred at 80°C for 16h. After the reaction was completed, the solvent was removed with a rotary evaporator, and the crude product was subjected to column chromatography, and the eluent was a mixed solvent of petroleum ether and ethyl acetate to obtain 55.1 mg of dihydronaphthalene derivative 3aa substituted with difluoromethyl, which was separated and collected The rate is 84%.
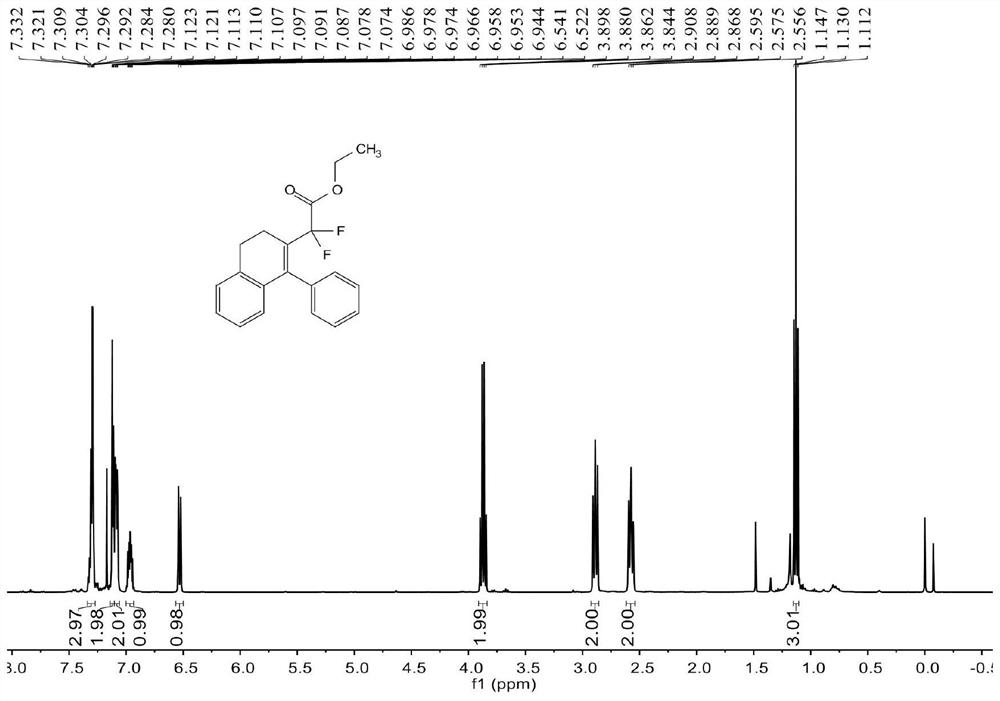
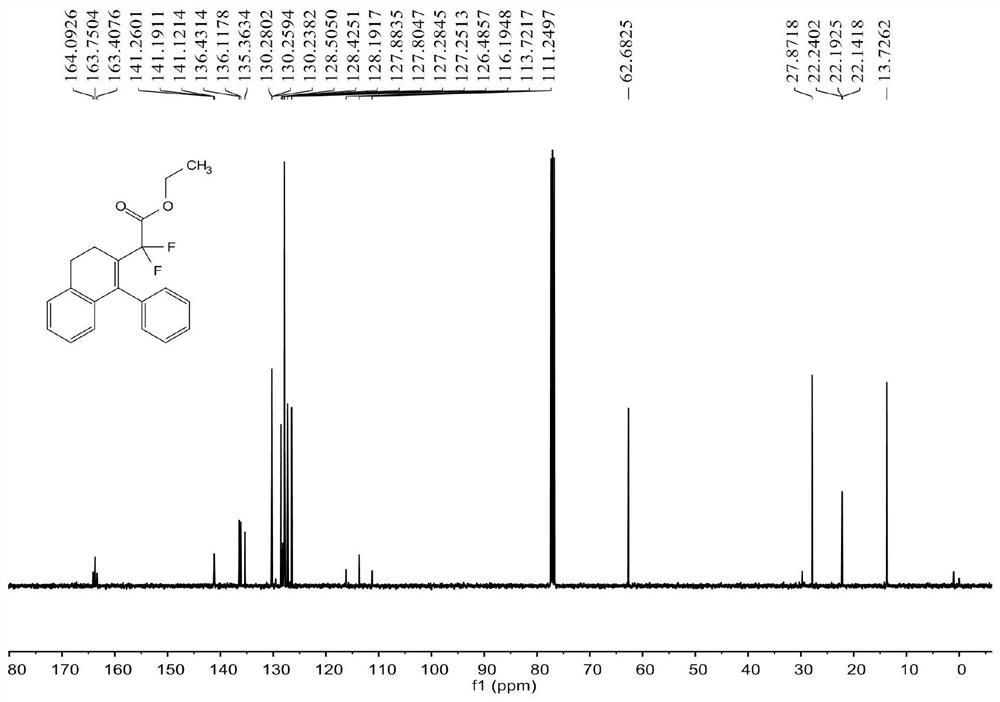
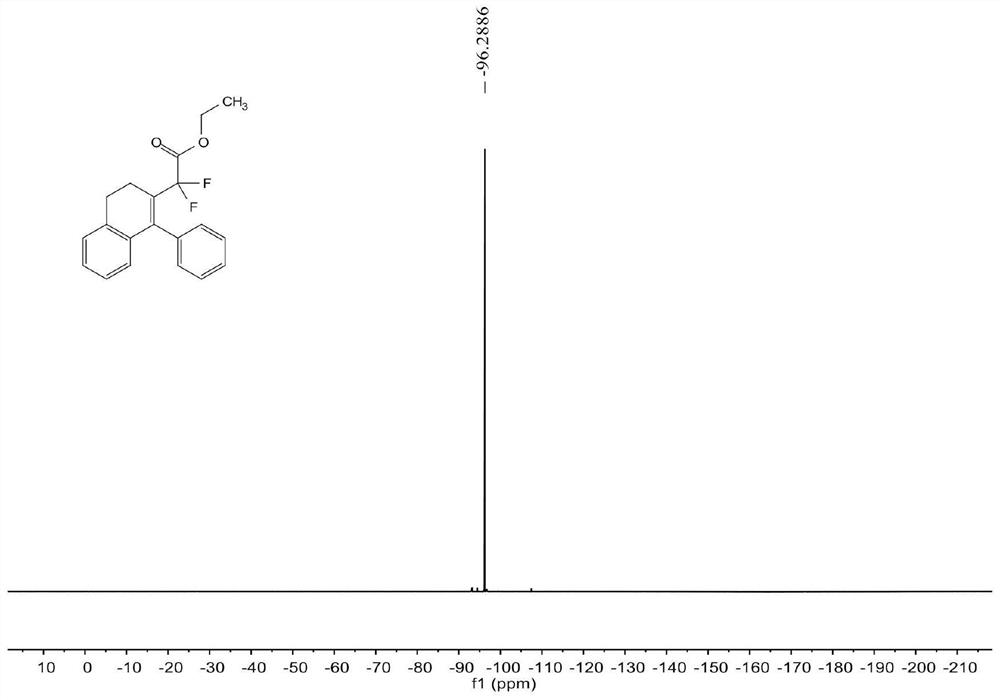
[0070] see Figure 1 ~ Figure 3 , the characterization data of compound 3aa are as follows:
[0071] 1 H NMR (400MHz, CDCl 3 )δ7.33-7.28(m,3H),7.12-7.11(m,2H),7.10-7.07(m,2H),6.99-6.94(m,1H),6.53(d,J=7.6Hz,1H) , 3.87(q, J=7.2Hz, 2H), 2.89(t, J=8.0Hz, 2H), 2.58(t, J=8.0Hz, 2H), 1.13(t, J=7.2Hz, 3H)...
Embodiment 2
[0073]
[0074] After vacuumizing the reactor, replace it with inert gas nitrogen or argon, add 0.2mmol (41.6mg) 1b, 0.4mmol (80.8mg) ethyl difluorobromoacetate (2a), 0.02mmol (2.9mg) CuBr, 0.02 mmol (7.1 mg) dtbbpy, 0.06 mmol (15.2 mg) B 2 pin 2 , 0.4 mmol (33.6 mg) NaHCO 3 , 1mL 1,4-dioxane, stirred at 80°C for 16h. After the reaction was finished, the solvent was removed with a rotary evaporator, and the crude product was subjected to column chromatography, and the eluent was a mixed solvent of sherwood oil and ethyl acetate to obtain 47.5 mg of dihydronaphthalene derivative 3ba substituted with difluoromethyl, which was separated and collected. The rate is 72%.
[0075] see Figure 4 ~ Figure 6 , the characterization data of compound 3ba are as follows:
[0076] 1 H NMR (400MHz, CDCl 3 )δ7.33(dd, J=8.0,2.0Hz,1H),7.30(s,1H),7.00(d,J=8.0Hz,1H),6.82(s,1H),4.35(q,J=7.2 Hz,2H),2.85(t,J=8.0Hz,2H),2.45-2.40(m,2H),1.35(t,J=7.2Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ163.6(t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com