A kind of cycloalkyl-containing β-hydroxyl sulfone compound and its synthesis method
A synthetic method and cycloalkyl-containing technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as low yield, achieve good substrate universality, step economy, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] This embodiment provides a method for synthesizing cycloalkyl-containing β-hydroxysulfone compounds. A cycloalkyl group with a relatively large tension can be introduced into the β-hydroxysulfone compounds. The method specifically includes the following steps:
[0040] Add methylene cycloalkane compounds, sulfonyl chloride, photocatalyst, base and solvent in sequence in the reactor, and react with stirring at room temperature under visible light irradiation;
[0041] After the reaction, the solvent was removed with a rotary evaporator to obtain a crude product, which was obtained through column chromatography, wherein the eluent was a mixed solvent of petroleum ether and ethyl acetate.
[0042] Preferably, the solvent in the above synthesis method is N,N-dimethylformamide, dimethyl sulfoxide, dichloromethane, acetonitrile, 1,4-dioxane, 1,2-dichloroethane, tetrahydrofuran, A mixture of one or more of ethanol or methanol with water. In the experiment, it was found that s...
Embodiment 1
[0055]
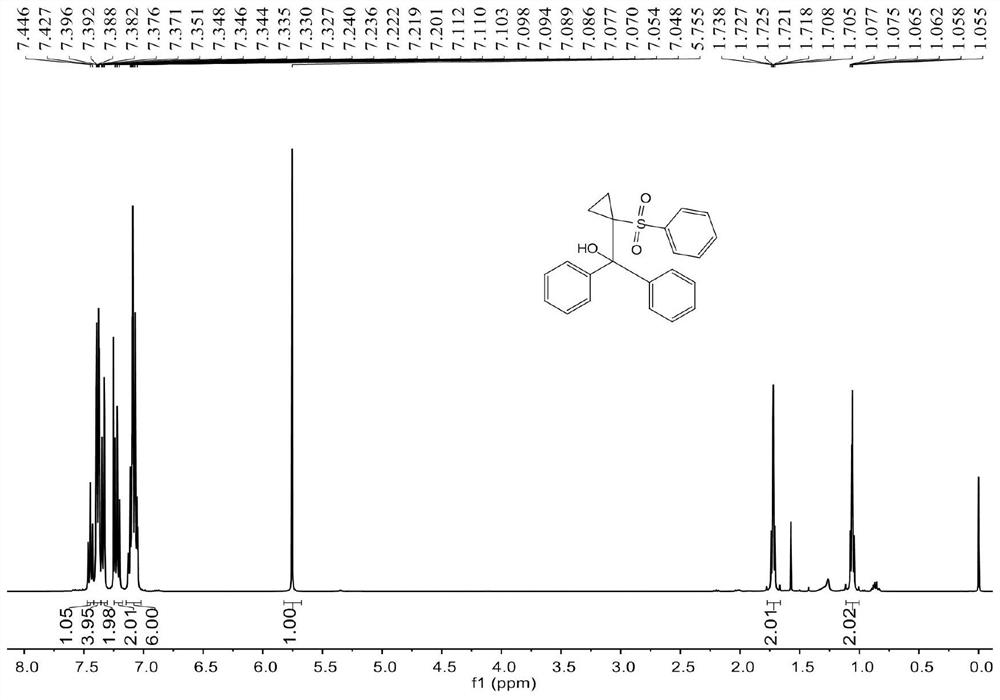
[0056] The reactor was placed under nitrogen, and 0.2mmol (41.2mg) methylenecyclopropane 1a, 0.3mmol (52.8mg) benzenesulfonyl chloride 2a, 2% (3.0mg) [Ru(bpy) 3 ]Cl 2 ·6H 2 O, 0.3 mmol (52.2 mg) K 2 HPO 4 , 1mL acetonitrile / water (30 / 1), under the irradiation of 12W blue light LEDs, stir at room temperature for 3h; The solvents were mixed to obtain 56.8 mg of cyclopropyl-containing β-hydroxysulfone compound 3aa (diphenyl(1-(phenylsulfonyl)cyclopropyl)methanol), with an isolated yield of 78%.
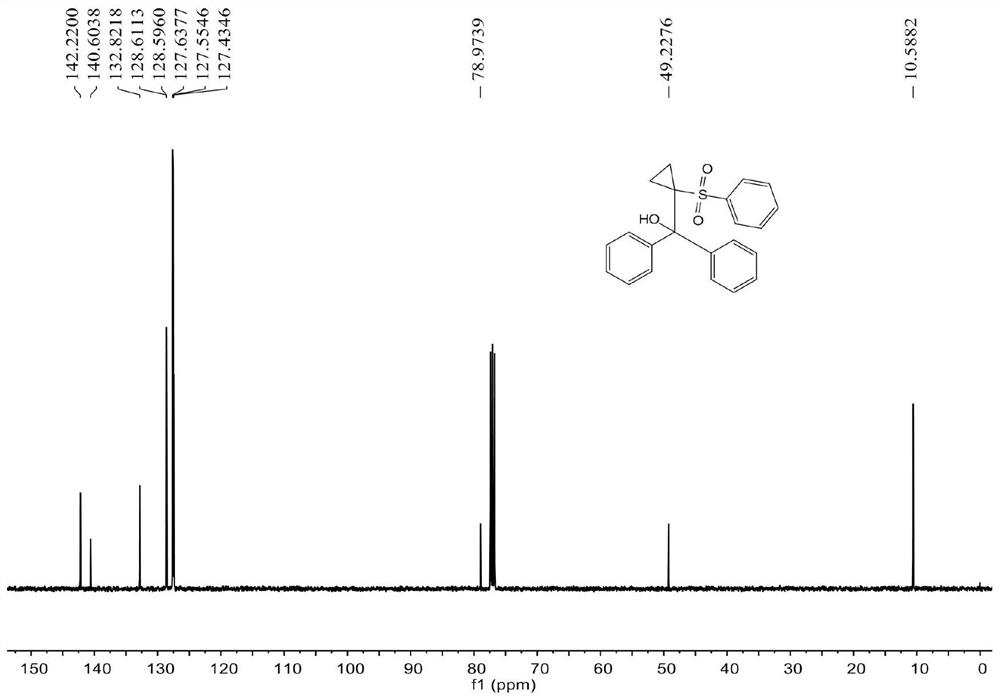
[0057] see figure 1 , figure 2 , the characterization data of compound 3aa are as follows:
[0058] 1 H NMR (400MHz, CDCl 3 )δ7.47-7.42(m,1H),7.40-7.37(m,4H),7.36-7.32(m,2H),7.24-7.20(m,2H),7.13-7.05(m,6H),5.76( s,1H),1.74-1.71(m,2H),1.08-1.04(m,2H). 13 CNMR (100MHz, CDCl 3 ) δ 142.2, 140.6, 132.8, 128.6, 128.5, 127.6, 127.5, 127.4, 79.0, 49.2, 10.6.
Embodiment 2
[0060]
[0061] The reactor was placed under nitrogen, and 0.2mmol (41.2mg) methylenecyclopropane 1a, 0.3mmol (57.2mg) p-toluenesulfonyl chloride 2b, 2% (3.0mg) [Ru(bpy) 3 ]Cl 2 ·6H 2 O, 0.3 mmol (52.2 mg) K 2 HPO 4 , 1mL acetonitrile / water (30 / 1), under the irradiation of 12W blue light LEDs, stir at room temperature for 3h; The solvents were mixed to obtain 68.0 mg of cyclopropyl-containing β-hydroxyl sulfone compound 3ab (diphenyl(1-tosylcyclopropyl)methanol), with an isolated yield of 90%.
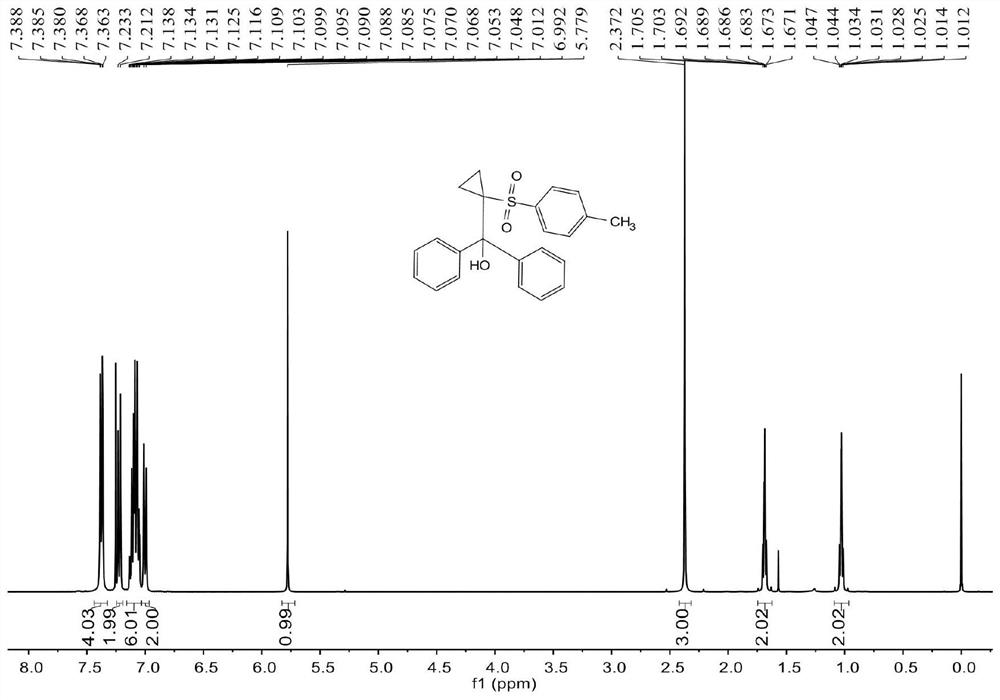
[0062] see image 3 , Figure 4 , the characterization data of compound 3ab are as follows:
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.39-7.36(m,4H),7.22(d,J=8.4Hz,2H),7.14-7.05(m,6H),7.00(d,J=8.0Hz,2H),5.78(s,1H ),2.37(s,3H),1.71-1.67(m,2H),1.05-0.91(m,2H). 13 C NMR (100MHz, CDCl 3 ) δ 143.8, 142.4, 137.5, 129.2, 128.6, 127.7, 127.5, 127.2, 79.0, 49.1, 21.5, 10.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com