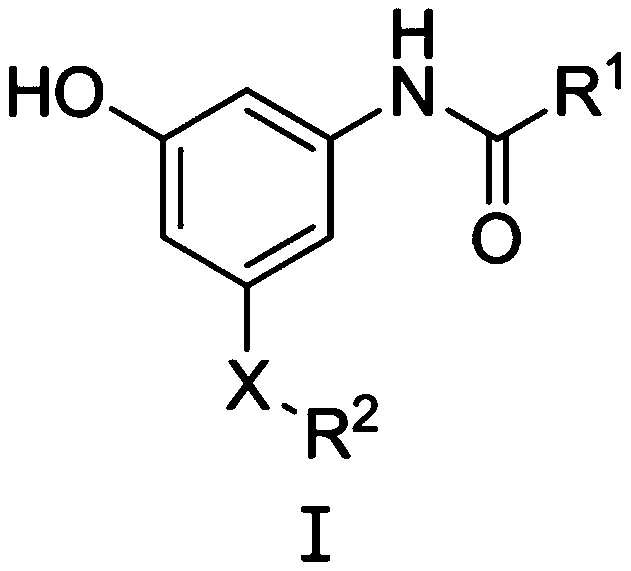
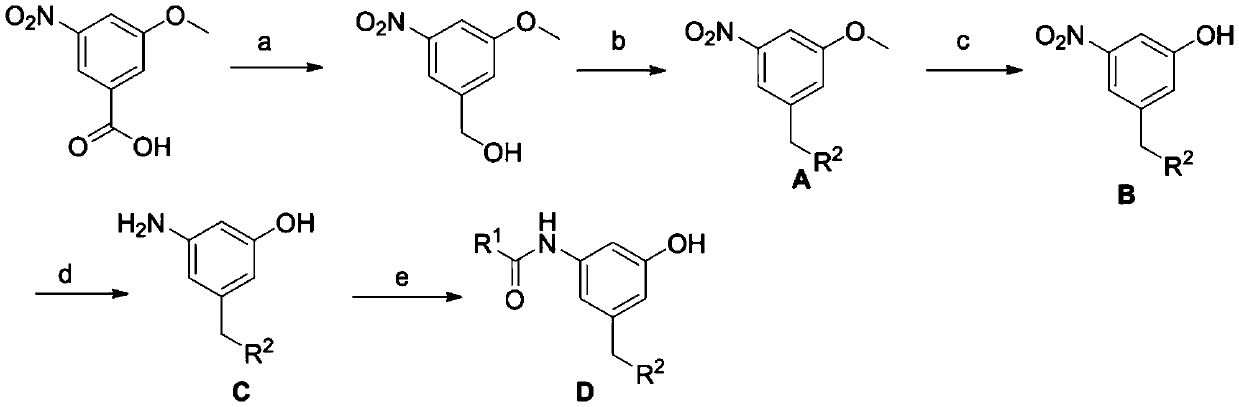
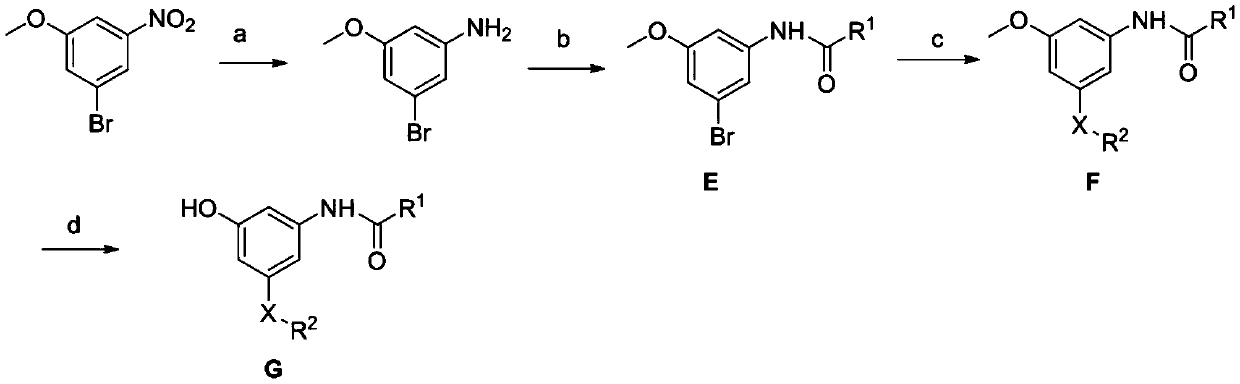
Meta-disubstituted phenol compound and its preparation method and anti-tuberculosis application
A phenol compound and a disubstituted technology, applied in the field of medicinal chemistry, can solve the problems of large side effects, unsuitable for long-term medication and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1 Synthetic N-(3-benzyl-5-hydroxyl phenyl)-2-phenylacetamide
[0079]
[0080] In a 100mL two-necked flask, sodium borohydride (1.6g, 42mmol) and redistilled tetrahydrofuran (20mL) were added under the protection of argon, and 3-nitro-5-methoxybenzoic acid (3.94 g, 20mmol), and then add boron trifluoride ether solution 6.7mL. After reacting at room temperature for 4 h, the reaction solution was quenched with dilute hydrochloric acid under ice cooling, and then extracted with dichloromethane (30 mL×2). The solvent of the extract was removed under reduced pressure to obtain 3.6 g of crude product 3-nitro-5-methoxybenzyl alcohol, which was directly used in the next reaction.
[0081] In a 100mL round bottom flask, add anhydrous aluminum trichloride (5.58g, 42mmol), 20mL of anhydrous benzene and 10mL of dichloromethane, slowly add 3-nitro-5-methoxybenzyl alcohol under ice-bath conditions (3.6g, 20mmol), and then reflux at 80°C for 5h. After cooling, the rea...
Embodiment 2
[0086] Embodiment 2 Synthetic N-(3-benzyl-5-hydroxyl phenyl) cyclohexyl formamide
[0087]
[0088] Refer to Example 1 for the synthesis of 1-benzyl-3-aminophenol. In a 50mL round bottom flask, add 1-benzyl-3-aminophenol (99.5mg, 0.5mmol), cyclohexanecarbonyl chloride (74μL, 0.55mmol), triethylamine (76μL, 0.55mmol) under ice cooling and redistilled tetrahydrofuran 2mL, reacted at room temperature for 12h, the reaction solution was extracted with ethyl acetate (15mL×2), and the solvent was removed from the extract under reduced pressure to obtain a crude product, which was purified by silica gel column chromatography (petroleum ether / ethyl acetate Ester gradient elution) to obtain 75 mg of white solid N-(3-benzyl-5-hydroxyphenyl)cyclohexanecarboxamide with a yield of 49%.
[0089] 1 H NMR(400MHz,DMSO)δ9.59(s,1H),9.24(s,1H),7.28(m,2H),7.19(m,3H),7.04(m,1H),6.84(m,1H) ,6.28(m,1H),3.77(s,2H),2.27(t,J=11.4Hz,1H),1.73(d,J=9.2Hz,4H),1.63(d,J=9.6Hz,1H) ,1.37(m,2H),1.30–1.14(m,...
Embodiment 3
[0090] Example 3 Synthesis of N-(3-benzyl-5-hydroxyphenyl)-2-(4,4-dimethylcyclohexyl)acetamide
[0091]
[0092] Refer to Example 1 for the synthesis of 1-benzyl-3-aminophenol. In a 25 mL round bottom flask, add 1-benzyl-3-aminophenol (160 mg, 0.8 mmol), 2-(4,4-dimethylcyclohexyl) acetic acid (204 mg, 1.2 mmol), triphenyl phosphite (314μL, 1.2mmol) and redistilled toluene 5mL were reacted at 110°C for 12h. The solvent was removed from the reaction solution under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / dichloromethane gradient elution) to obtain a white solid, namely N-(3-benzyl-5-hydroxyphenyl)-2-(4 , 4-dimethylcyclohexane) acetamide 127mg, yield 45%.
[0093] 1 H NMR(400MHz,DMSO)δ9.60(s,1H),9.21(s,1H),7.28(m,2H),7.18(m,3H),7.06(m,1H),6.79(m,1H) ,6.28(m,1H),3.78(s,2H),2.15(d,J=7.1Hz,2H),1.64(m,1H),1.49(m,2H),1.33(m,2H),1.19– 1.10(m,4H),0.87(s,3H),0.86(s,3H). 13C NMR (101MHz, DMSO) δ170.89, 157.99, 142.94, 141...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com