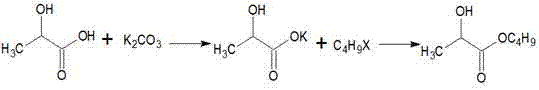
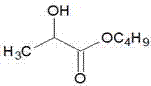
Method for synthesizing high-purity butyl lactate
A technology of butyl lactate and a synthesis method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of carboxylate, etc., can solve the problems of complicated preparation process, unsuitable for large-scale production, etc., and achieves simple operation and low cost. , the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 200mL of water and 180.2g (2mol) of lactic acid into a 1000mL three-neck flask, stir, add dropwise an aqueous solution prepared by 84g (2.1mol) of sodium hydroxide at 20°C, and control the temperature of natural reaction not to exceed 90°C. After the addition is complete, continue Stir for 0.5 hours, distill under reduced pressure to remove water to dryness, cool down to 20°C, add 301.5 g (2.2 mol) of n-bromobutane dropwise, keep the reaction for 10 hours, add 600 mL of water to wash twice, and rectify the organic layer to obtain the product 282 grams, the gas chromatography content is 99.6%, and the molar yield is 96.5% based on lactic acid.
Embodiment 2
[0021] Add 200mL and 180.2g (2mol) of lactic acid into a 1000mL three-necked flask, stir, add dropwise an aqueous solution prepared by 138.3g (1mol) of potassium carbonate at 20°C, control the temperature of natural reaction not to exceed 90°C, and continue stirring for 1 hour after the dropwise addition is completed , distilled under reduced pressure to remove water to dryness, lowered the temperature to 20°C, added 274.1 g (2 mol) of n-bromobutane dropwise, raised the temperature to 40°C and kept the temperature for 8 hours, added 600mL water to wash twice, and rectified the organic layer to obtain the product 285.1 grams, the gas chromatography content is 99.5%, and the molar yield is 97.5% based on lactic acid.
Embodiment 3
[0023] Add 200mL and 180.2g (2mol) of lactic acid into a 1000mL three-necked flask, stir, add dropwise an aqueous solution prepared by 220.2g (2.2mol) of potassium bicarbonate at 20°C, and control the temperature of natural reaction not to exceed 90°C. After the addition is complete, continue stirring After 2 hours, remove water by distillation under reduced pressure to dryness, cool down to 20°C, add 274.1 g (2 mol) of brominated n-butane dropwise, raise the temperature to 60°C and keep the reaction for 5 hours, cool down to below 50°C, add 600mL water to wash twice, The organic layer was rectified to obtain 284.5 grams of the product, with a gas chromatographic content of 99.2%, and a molar yield of 97.3% based on lactic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com