PH (potential of hydrogen) controlled-release targeted medicine nanometer delivery carrier, method for preparing same and application of pH controlled-release targeted medicine nanometer delivery carrier
A nano and carrier technology, applied in the direction of drug delivery, pharmaceutical formulation, drug combination, etc., can solve the problems of complex synthesis method, single targeting and high cost of organic coating agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1pH controlled-release targeted drug nano-transport carrier Fe 3 o 4 @mSiO 2 -FA-DNM-CaCO 3 Preparation of Nanospheres
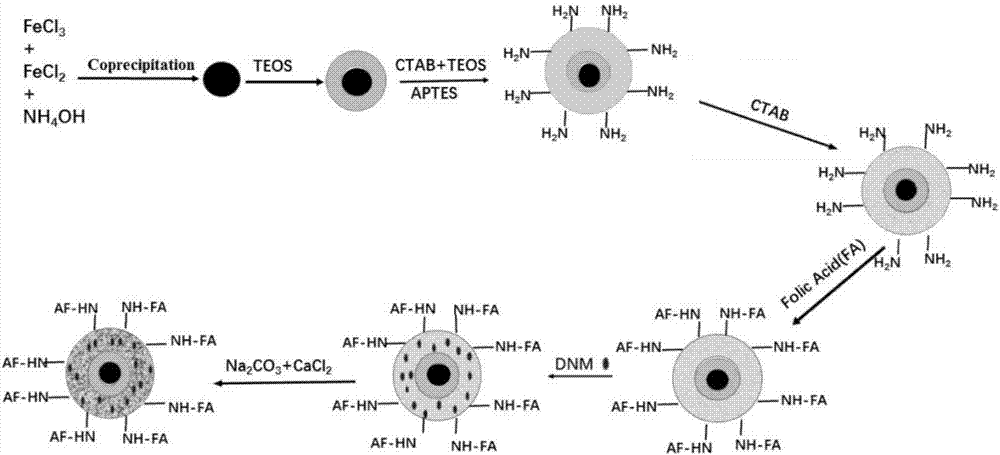
[0099] The specific preparation process can refer to figure 1 , the process is as follows:
[0100] Step 1: Fe 3 o 4 Preparation of Nanospheres
[0101] First weigh 1.99g FeCl 3 .6H 2 O and 0.99 g FeCl 2 .7H 2 O(Fe 2+ : Fe 3+ The molar ratio is about 2:1, Fe 2+ A slight excess of Fe 2+ easy to be oxidized) in a 250mL round bottom flask, blow nitrogen for a while, add 100ml deoxygenated deionized water, stir to dissolve, heat to 80°C in a heating oil bath, stir at 6.5krp / min, quickly add The newly prepared ammonia water (take 12mL of 25% concentrated ammonia water and dilute to 50mL), test the pH range with pH test paper, and keep the reaction pH value at 10-11; the liquid turns black immediately, and continue to react for 30min; after the reaction is completed, use a magnet Adsorption separation Adsorb the magnetic particles to...
Embodiment 2
[0120] The experiment of the amount of embodiment 2 immobilized daunomycin (DNM)
[0121] Get the Fe obtained in 10mg embodiment 1 step 4 3 o 4 @mSiO 2 - Add 5ml of DNM solution to FA nanospheres in a test tube, the concentration of the DNM solution is 0.14, 0.33, 0.49, 0.65, 0.86 mg / ml, place on a shaker (180rp / min) for 24 hours, and then centrifuge Remove the supernatant, measure the concentration of the supernatant, calculate the immobilization rate, and find out the optimal immobilization concentration. The specific experimental results are as Figure 10 shown.
[0122] from Figure 10 It can be seen that as the concentration of the DNM solution increases, the immobilization rate also increases accordingly, and the increase rate of the immobilization rate has tended to be parallel when the concentration is 0.86mg / ml. fixed load.
Embodiment 3
[0123] Embodiment 3 Sustained release experiment
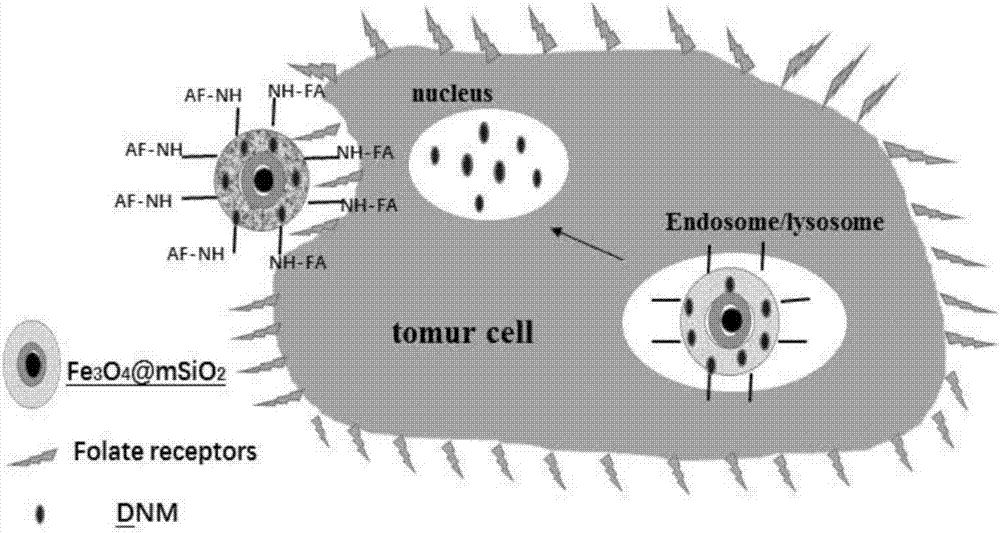
[0124] Will Fe 3 o 4 @mSiO 2 -FA-DNM nanospheres and Fe 3 o 4 @mSiO 2 -FA-DNM-CaCO 3 The nanospheres are respectively placed in test tubes containing 10 ml of PBS buffer solution, the pH of which is 5.6 and 7.4; Take out 3ml release solution within the time (1, 2, 4, 6h...), and then add 3ml fresh PBS buffer solution respectively, and measure the cumulative release amount under the conditions of PBS buffer solution with pH=5.6 and pH=7.4. Specific results such as Figure 7 shown.
[0125] from Figure 7 It can be seen that Fe 3 o 4 @mSiO 2 -FA-DNM nanospheres and Fe 3 o 4 @mSiO 2 -FA-DNM-CaCO 3 The cumulative release of nanospheres under the PBS buffer solution condition of pH=5.6 is more than that under the PBS buffer solution condition of pH=7.4; under the same PBS buffer solution condition, Fe 3 o 4 @mSiO 2 - Cumulative release of FA-DNM nanospheres compared to Fe 3 o 4 @mSiO 2 -FA-DNM-CaCO 3 There ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com