Methods and antibodies for modulation of immunoresponse
An antibody, reactive technology, applied in the field of immunotherapy, which can solve the problem of mutual influence without prompting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0186] Materials and methods used in the following examples are described below:
[0187] Materials and methods
[0188] Human Cell Isolation and Cell Culture
[0189] Leukocyte concentrates from healthy volunteers were obtained from the Taiwan Blood Service Foundation (Taipei, Taiwan). Written informed consent was obtained for participation in the study, which was approved by the Institutional Review Board of the Mackay Memorial Hospital. Human monocytes were isolated as previously described. Briefly, peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Paque Plus (GE Healthcare) gradient centrifugation. These monocytes were further purified by CD14 selection using CD14MACS microbeads (Miltenyi Biotec). The purity of the monocytes was confirmed to be approximately 90% using flow cytometry analysis.
[0190] Animal and tumor cell lines.
[0191] C57BL / 6 mice (6 to 8 weeks old) were purchased from the Experimental Animal Center (Taipei, Taiwan). All anima...
example 1
[0210] Example 1: Binding CD11b will reduce PD-L1 expression on LPS-sensitized monocytes
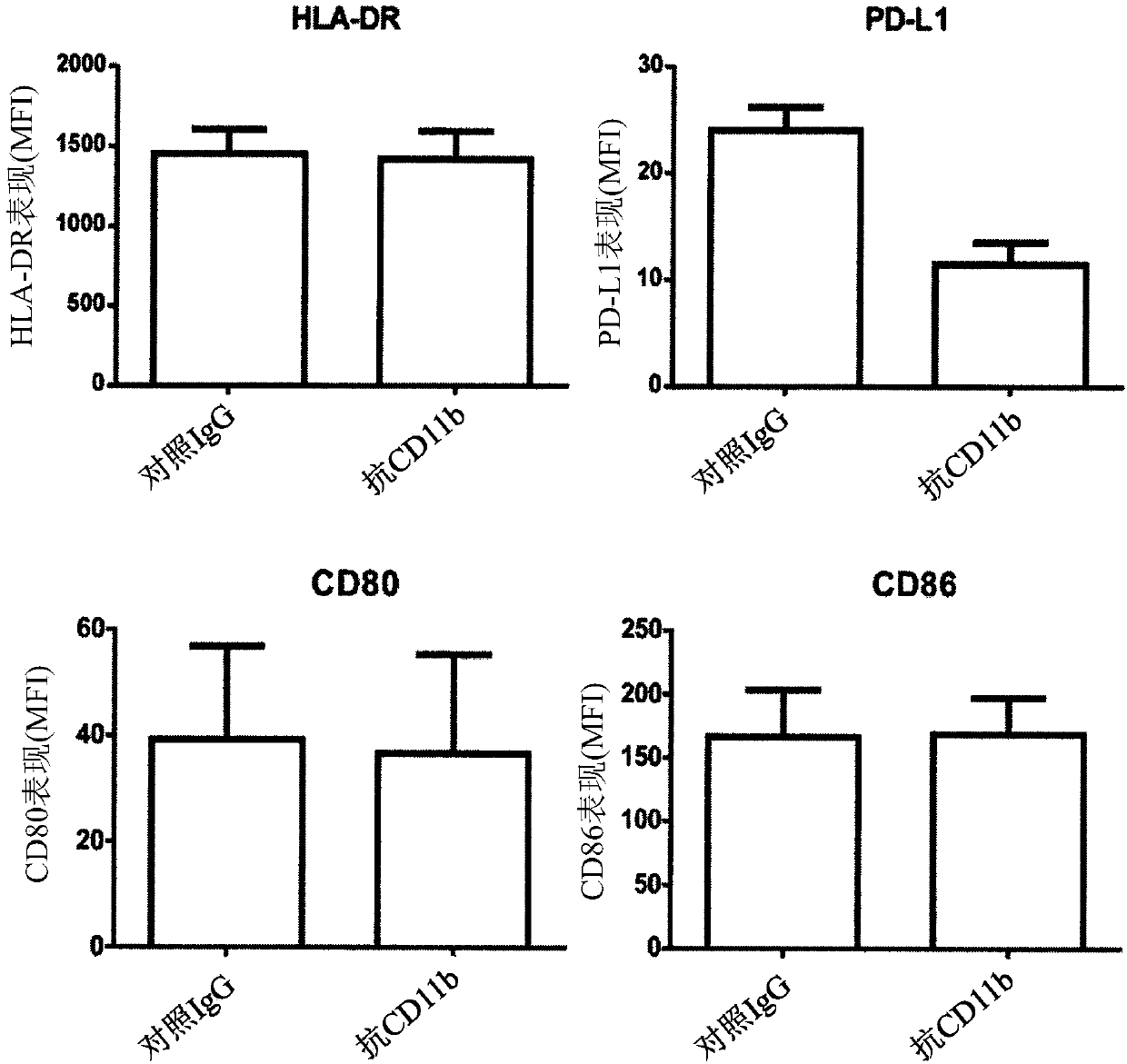
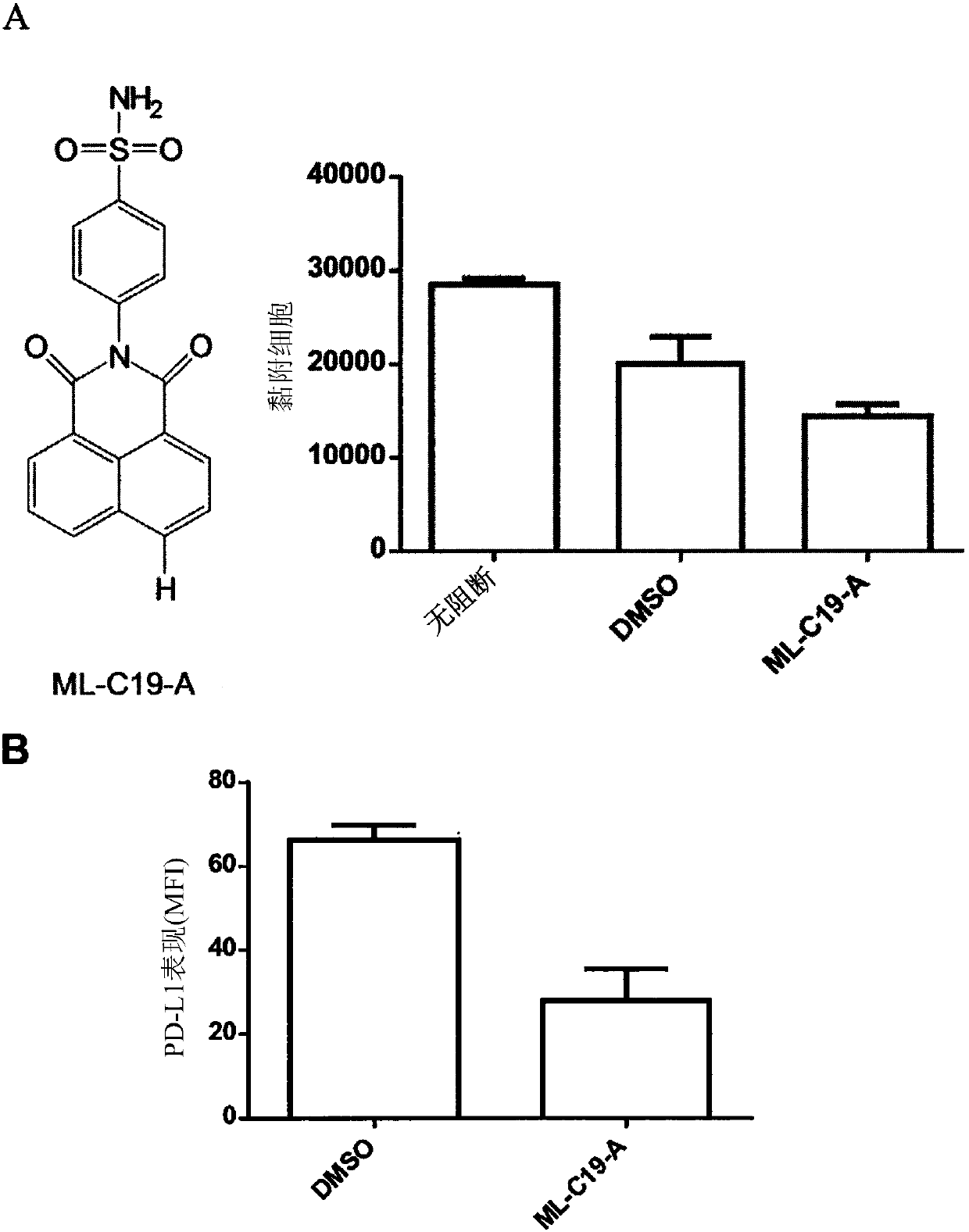
[0211] In this example, we investigated whether blockade of integrin αMβ2 (Mac-1) could functionally increase TLR responses. Such as figure 1 Administration of a CD11b-binding agent, such as an anti-CD11b antibody (ICRF44), reduces LPS-induced PD-L1 expression on monocytes, as shown in . In contrast, anti-CD11b antibody treatment did not alter the expression levels of HLA-DR, CD80, and CD86 on LPS-sensitized monocytes. Take ML-C19-A (a small molecule of CD11b antagonist) ( figure 2 A) Binding to CD11b also confirmed the inhibition of PD-L1 expression in LPS-sensitized monocytes ( figure 2 B). Together these results suggest that CD11b plays a critical role in the induction of PD-L1 expression on LPS-sensitized monocytes.
example 2
[0212] Example 2: Effects of CD11b Binding in Antitumor Immunity
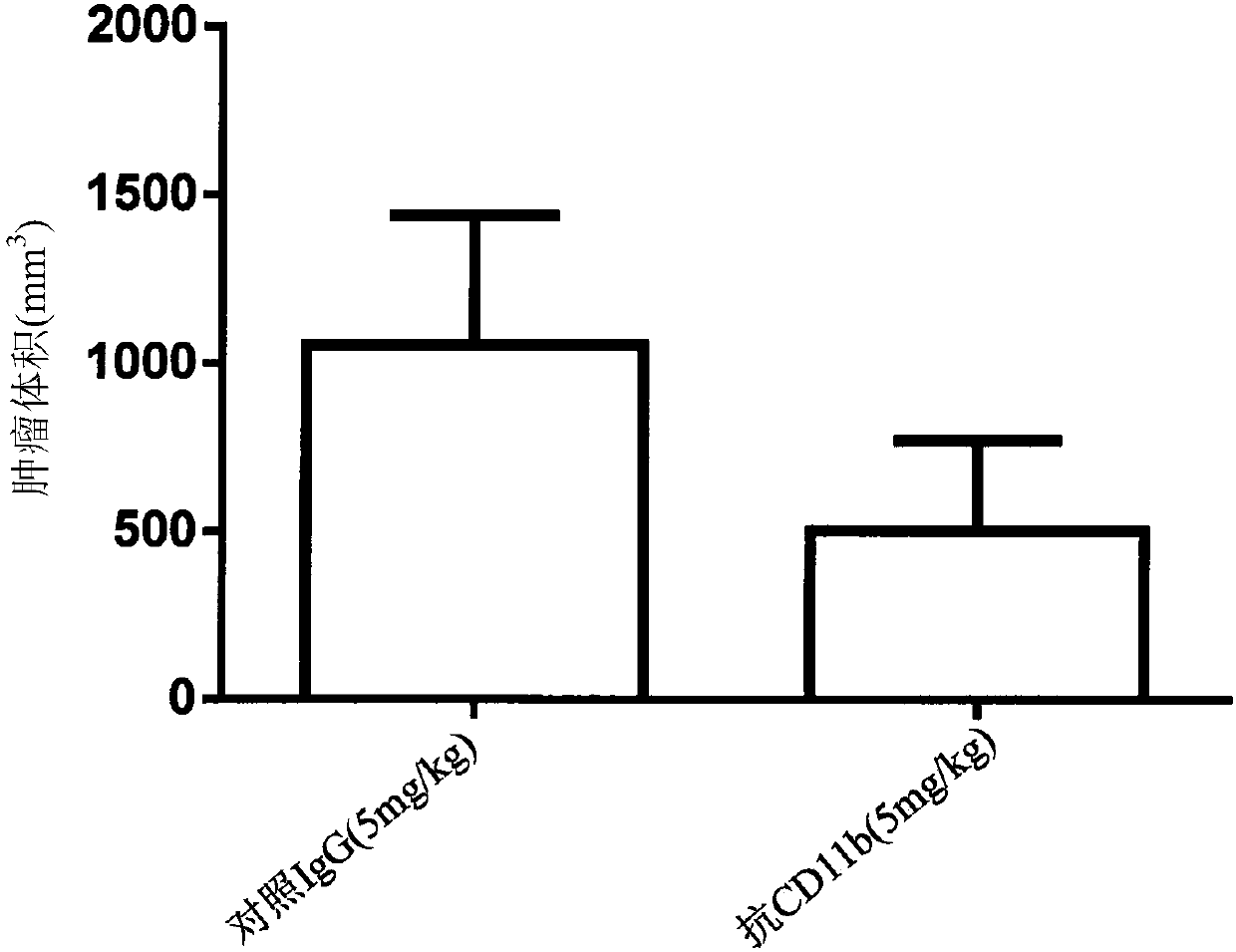
[0213] To test the effect of CD11b binding in anti-tumor immunity, an anti-mouse CD11b(M1 / 70) antibody was tested as monotherapy in the B16F10 murine tumor model. C57BL / 6 mice were subcutaneously injected with B16F10 cells on day 0. On day 7, mice were injected intraperitoneally (ip) with control IgG (5 mg / kg) or anti-mouse CD11b antibody (5 mg / kg). Repeat the injection every three to four days. Efficacy was determined by monitoring tumor volume and long-term survival for each group. Such as image 3 As shown in , binding to CD11b with an anti-mouse CD11b antibody potently inhibited the subcutaneous growth of B16F10 tumors (at day 18, control IgG compared to anti-CD11b=1054±385.4mm 3Compared to 502.7±268.2mm 3 ). We examined the proportion of the immune cell population in the tumor. On day 18 after tumor inoculation, binding of CD11b with an anti-CD11b antibody reduced the local accumulation of tumor-inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com