Organic electroluminescent device
An electroluminescent device and luminescent technology, applied in the direction of electric solid-state devices, electrical components, luminescent materials, etc., can solve the problems of insufficient current efficiency, low driving voltage, and insufficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196]
[0197] Under a nitrogen atmosphere, 3.0 g of 2,8-dibromodibenzofuran, 8.4 g of (4'-diphenylamino-biphenyl-4-yl)-phenylamine, 2.7 g of sodium tert-butoxide, and toluene 60ml was added to the reaction vessel, followed by adding tert-butylphosphine 0.2g, Pd 2 (dba) 3 0.2 g and heated, stirring at reflux for 10 hours. 30 ml of toluene was added, and the filtrate obtained by filtration was concentrated. After the concentrate was purified by column chromatography (carrier: silica gel, eluent: toluene / cyclohexane), N,N'-bis(4 '-diphenylamino-biphenyl-4-yl)-N,N'-diphenyl-dibenzofuran-2,8-diamine (compound 2) light yellow powder 7.6g (yield: 83.5%).
[0198]
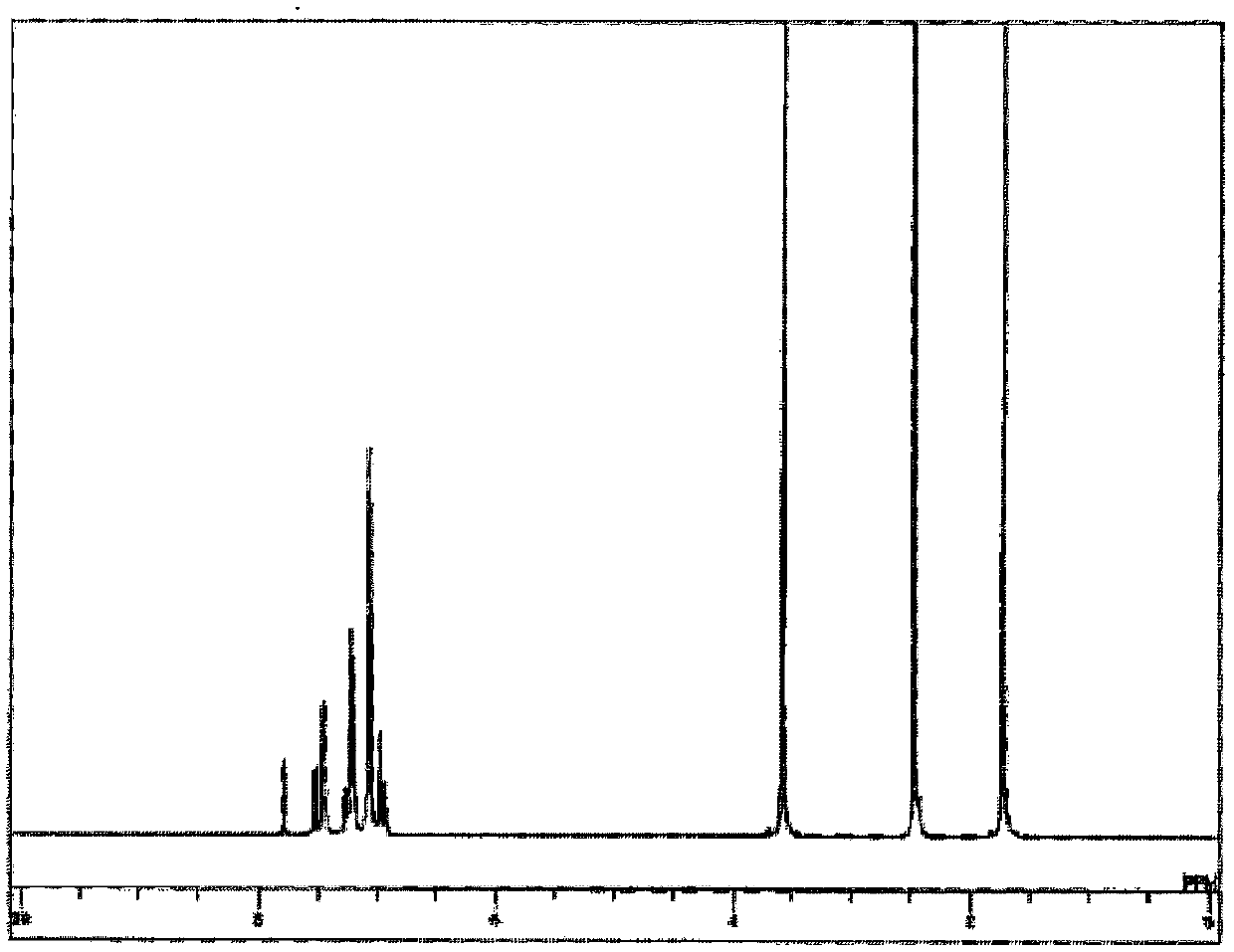
[0199] For the obtained pale yellow powder, NMR was used for structural identification. Will 1 H-NMR measurement results are shown in figure 1 .
[0200] pass 1 H-NMR (THF-d 8 ) detected the following 52 hydrogen signals. δ(ppm)=7.78(2H), 7.53(2H), 7.45(8H), 7.27(2H), 7.24-7.19(12H), 7.07(20H), 6.98(4H),...
Embodiment 2
[0202]
[0203] Under a nitrogen atmosphere, 5.0 g of 4,6-diiododibenzofuran, 11.8 g of (4'-diphenylamino-biphenyl-4-yl)-phenylamine, 4.9 g of potassium carbonate, hydrogen sulfite 0.4 g of sodium, 0.3 g of 3,5-di-tert-butylsalicylic acid, 10 ml of xylene, 5 ml of dodecylbenzene, and 0.1 g of copper powder were added to the reaction vessel, heated, and stirred at 210° C. for 12 hours. 60 ml of toluene was added, and the filtrate obtained by filtration was concentrated. After the concentrate was purified by column chromatography (carrier: silica gel, eluent: toluene / cyclohexane), N,N'-bis(4 '-Diphenylamino-biphenyl-4-yl)-N,N'-diphenyl-dibenzofuran-4,6-diamine (compound 33) white powder 9.3g (yield: 78.9 %).
[0204]
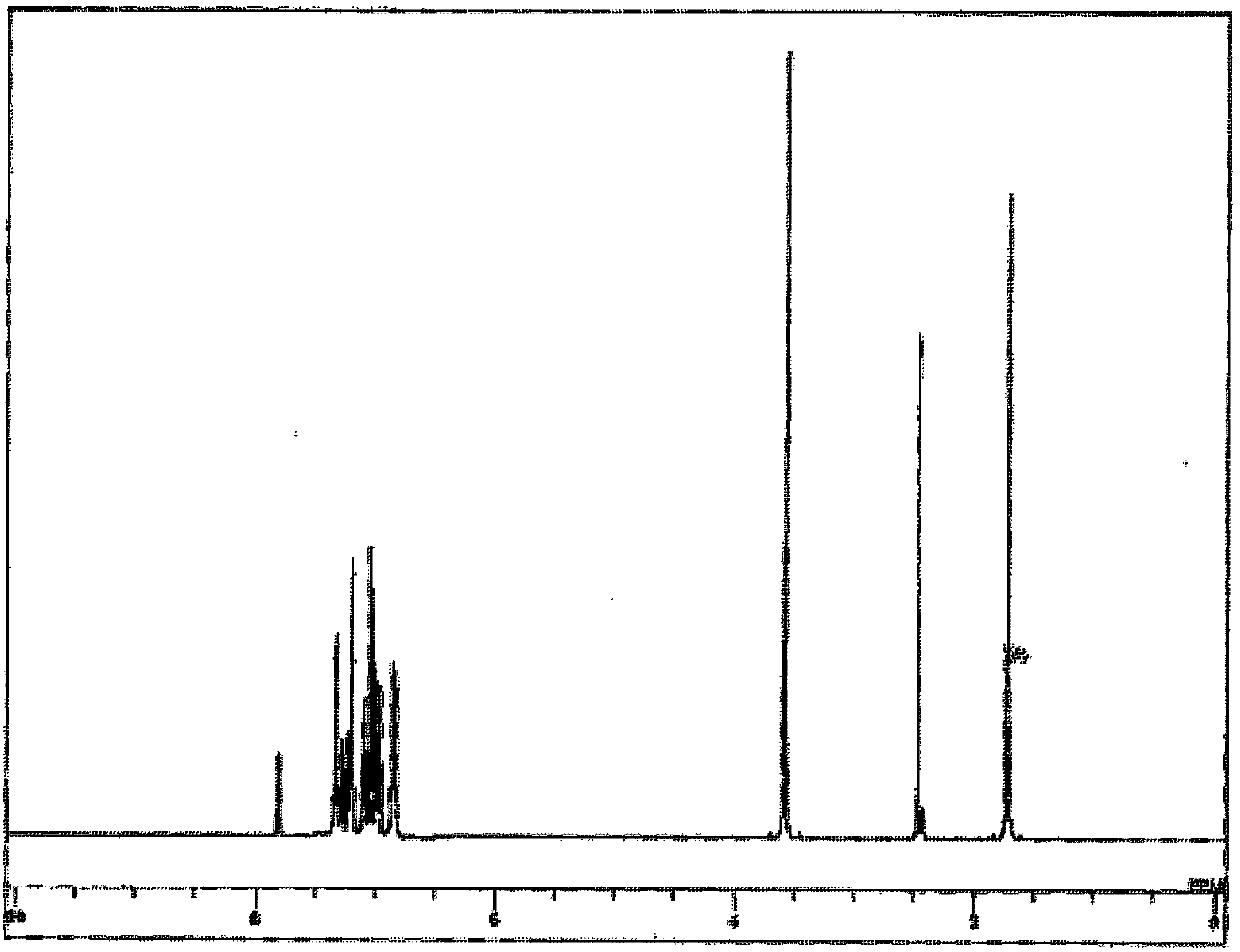
[0205] For the obtained pale yellow powder, NMR was used for structural identification. Will 1 H-NMR measurement results are shown in figure 2 .
[0206] pass 1 H-NMR (THF-d 8 ) detected the following 52 hydrogen signals. δ(ppm)=7.82(2H), 7.33(8H), ...
Embodiment 3
[0208]
[0209] Under a nitrogen atmosphere, 5.0 g of 2,8-dibromodibenzothiophene, 13.3 g of (4'-diphenylamino-biphenyl-4-yl)-phenylamine, 4.2 g of sodium tert-butoxide, and toluene 100ml was added to the reaction vessel, followed by adding 0.3g of tert-butylphosphine, Pd 2 (dba) 3 0.3 g and heated, stirring at reflux for 5.5 hours. After adding 50 ml of water and cooling to room temperature, the precipitated solid was obtained by filtration. The obtained solid was recrystallized from dichlorobenzene, thereby obtaining N,N'-bis(4'-diphenylamino-biphenyl-4-yl)-N,N'-diphenyl-dibenzo 11.3 g of light yellow powder of thiophene-2,8-diamine (compound 56) (yield: 76.9%).
[0210]
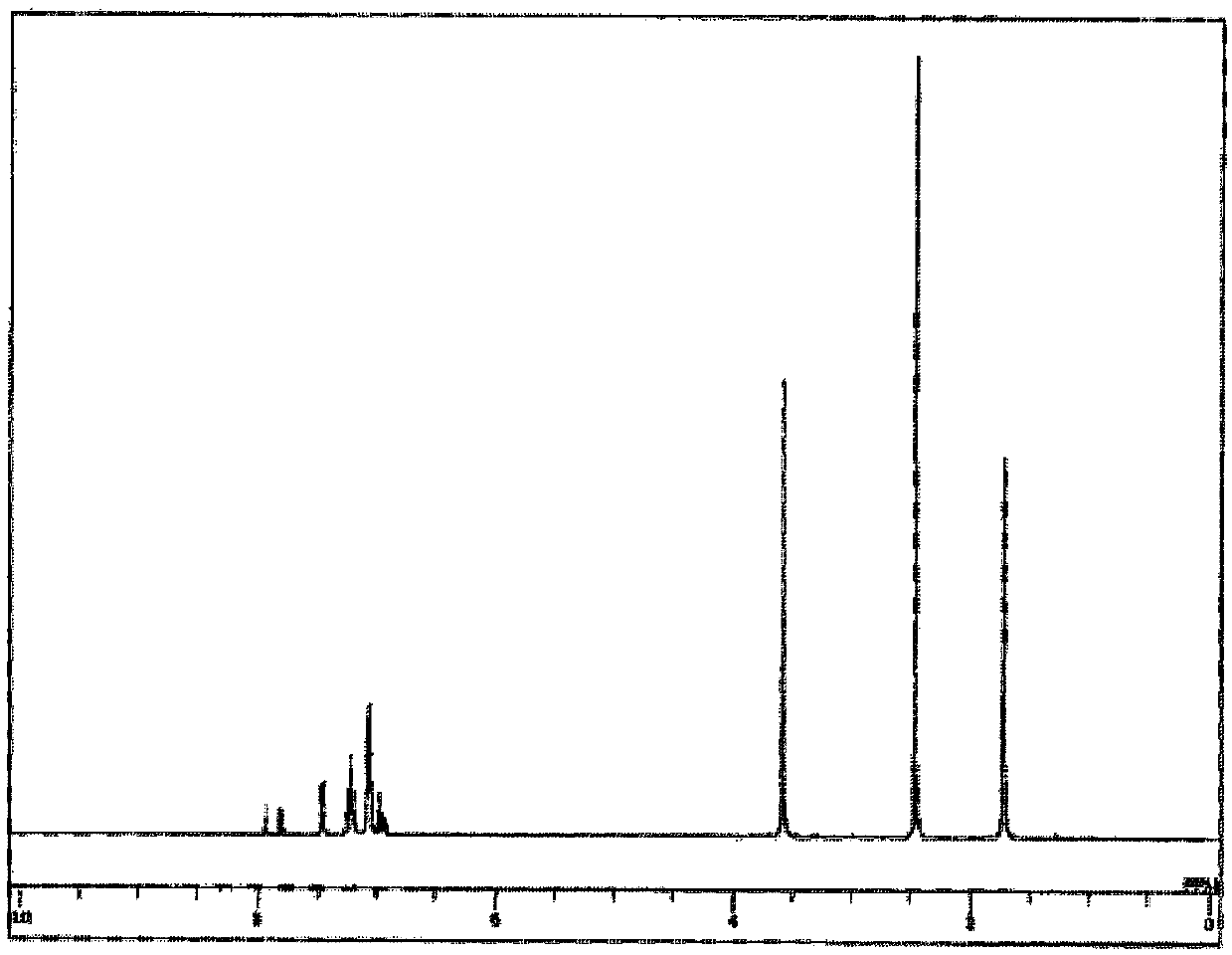
[0211] For the obtained white powder, NMR was used for structural identification. Will 1 H-NMR measurement results are shown in image 3 .
[0212] pass 1 H-NMR (THF-d 8 ) detected the following 52 hydrogen signals. δ(ppm)=7.94(2H), 7.81(2H), 7.46(4H), 7.45(4H), 7.25-7.19(14H), 7.08-7.05(20H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com