Catalyst for preparing pyromellitic dianhydride through durene gas phase oxidation
A technology of pyromethylene and gas-phase oxidation, which is applied in physical/chemical process catalysts, organic chemistry, chemical instruments and methods, etc., can solve the problems of low yield of mono-anhydride and high yield of mono-anhydride, and achieve improved activity and stability. , the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
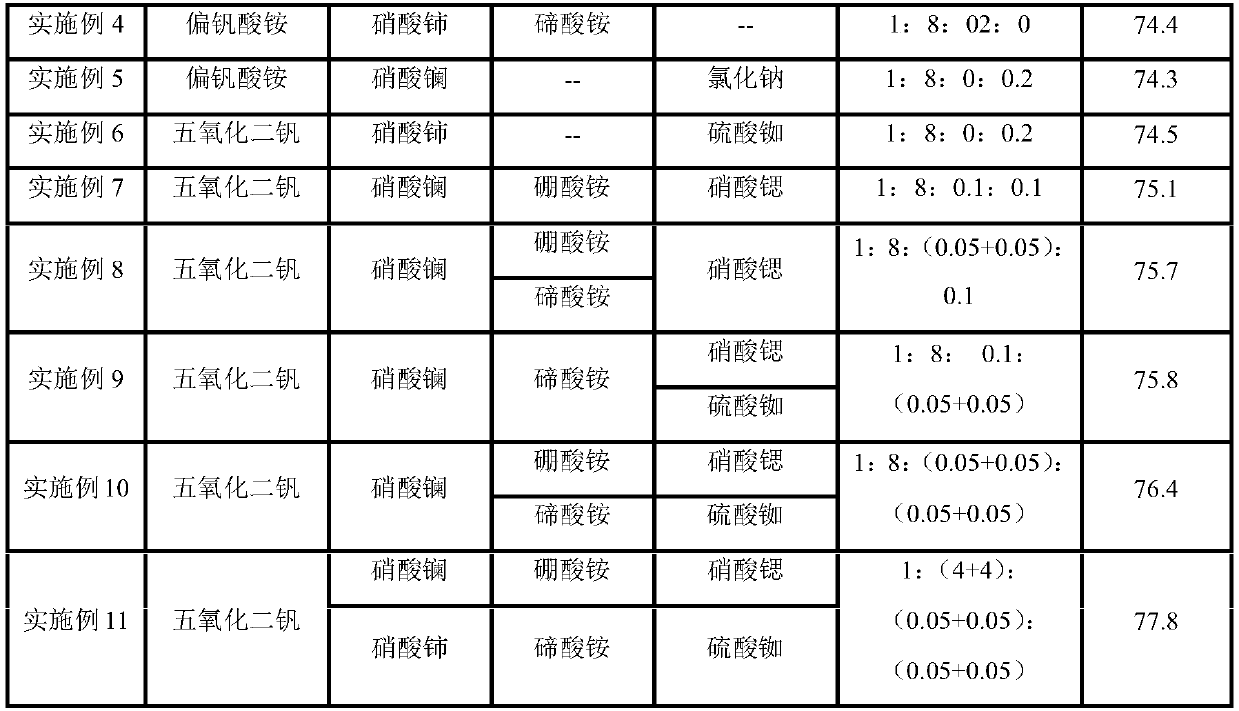
Examples
Embodiment 1
[0025] Weigh 95g of oxalic acid and 380ml of distilled water in a flask, stir and raise the temperature to 85°C, after the oxalic acid is completely dissolved, make an oxalic acid solution. Add 1 part of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. Add 8 parts of lanthanum nitrate and 0.2 parts of ammonium borate into the solution, and continue stirring evenly to obtain a catalyst precursor. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier silicon carbide. The inert carrier sprayed with the catalyst precursor was baked in a muffle furnace at 530°C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 525°C and a space velocity of 5150h -1 Next, it was evaluated in a fixed-bed reactor, and the average anhydride yield was measured to be 74.0%. The evaluation results are shown in Table...
Embodiment 2
[0027] Weigh 95g of oxalic acid and 380ml of distilled water in a flask, stir and raise the temperature to 85°C, after the oxalic acid is completely dissolved, make an oxalic acid solution. Add 1 part of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. Add 8 parts of lanthanum nitrate and 0.2 parts of strontium nitrate into the solution, and continue stirring evenly to obtain a catalyst precursor. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier silicon carbide. The inert carrier sprayed with the catalyst precursor was baked in a muffle furnace at 530°C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 525°C and a space velocity of 5150h -1 Next, it was evaluated in a fixed-bed reactor, and the average anhydride yield was measured to be 73.9%. The evaluation results are shown in Tab...
Embodiment 3
[0032] Weigh 95g of oxalic acid and 380ml of distilled water in a flask, stir and raise the temperature to 85°C, after the oxalic acid is completely dissolved, make an oxalic acid solution. Add 1 part of vanadium pentoxide to the prepared oxalic acid solution and continue stirring to obtain ammonium vanadyl oxalate solution. Add 8 parts of cerium nitrate and 0.2 parts of ammonium tellurate into the solution, and continue stirring to obtain a catalyst precursor. After the catalyst precursor is filtered and dried, it is loaded into a spraying machine and evenly sprayed on the inert carrier silicon carbide. The inert carrier sprayed with the catalyst precursor was baked in a muffle furnace at 530°C, and the catalyst was obtained after natural cooling. Catalyst at a reaction temperature of 525°C and a space velocity of 5150h -1Next, it was evaluated in a fixed-bed reactor, and the average anhydride yield was measured to be 74.2%. The evaluation results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com